Abstract
Background and Objectives
The aim of this study was to assess mechanical valve function using 64-slice multidetector computed tomography (MDCT).
Subjects and Methods
In 20 patients (mean age, 50±12 years; male-to-female ratio, 10:10), 30 St. Jude bileaflet mechanical valves (15 aortic and 15 mitral valves) were evaluated using MDCT. We selected images vertical and parallel to the mechanical valve. The valve orifice area (OA) and valve length were determined by manual tracing and the opening and closing angles were measured using a protractor. The OA and length of the mechanical valves were compared with the manufacturer's values.
Results
The geometric orifice areas (GOAs) based on the manufacturer's values and the OAs determined by MDCT were 3.4±0.2 cm2 and 3.4±0.3 cm2 for the mitral valves and 2.1±0.3 cm2 and 2.1±0.4 cm2 for the aortic valves, respectively. The correlation coefficients between the OA measures were 0.433 for the mitral valves and 0.874 for the aortic valves (both p<0.001). The lengths based on the manufacturer's values and determined by MDCT were 29.3±1.99 mm and 29.6±1.65 mm for the mitral valves and 21.5±2.1 mm and 20.7±2.3 mm for the aortic valves, respectively. The correlation coefficients between the measures were 0.651 for the mitral valve and 0.846 for the aortic valve (both p<0.001). The opening and closing angles determined by MDCT were 10.9±0.6° and 131.1±3.2° for the mitral valves and 11.1±0.9° and 120.6±1.7° for the aortic valves, respectively.
Traditionally, after mechanical valve replacement, transthoracic Doppler echocardiography (TTE) has been considered the diagnostic standard method for assessing and establishing mechanical valve (MV) function.1) However, an assessment of MV function by TTE has limitations, such as excessive metallic artifacts and a poor echo window in obese patients. Further, TTE is of limited value for patients with chronic obstructive pulmonary disease and for the evaluation of a MV in the aortic position.2) Even though transesophageal Doppler echocardiography (TEE) can provide better image quality,3) TEE is a semi-invasive method and can show metallic artifacts. It is difficult to obtain a perpendicular image of the MV with a fluoroscopic examination4) and an accurate measurement of the opening angle is difficult to obtain.4-8) Recently, the use of 64-slice multidetector computed tomography (MDCT) has been shown to be valuable for the measurement of coronary artery stenoses,9-12) determination of coronary calcium scores,13) measurement of left ventricular ejection fractions,14) follow-up of percutaneous coronary intervention,15)16) and follow-up of coronary artery bypass grafts.17-20) The aim of this study was to assess MV function using 64-slice MDCT.
This prospective study was performed on 20 patients between 1 March and 31 August 2006 at St. Mary's Hospital of The Catholic University of Korea in Seoul, Korea. The patients received 30 St. Jude medical (SJM) valves (bileaflet mechanical valves: 15 aortic and 15 mitral valves; St. Jude Medical, Inc., Minneapolis, MN, USA). Patients were enrolled in the study if they met all of the following inclusion criteria: 1) a previous mitral valve replacement performed at St. Mary's Hospital; 2) normal sinus rhythm {mean heart rate, 69 beats per minute (bpm); range, 50-97 bpm} as measured by an electrocardiogram; 3) a poor echo window, as seen on TTE; 4) refusal to undergo TEE, and 5) if in a clinically stable condition, the patient was able to hold his/her breath for 15 seconds. The Institutional Review Committee of our hospital approved this study. The subjects were informed of the investigative nature of the study and written consent was obtained before entry.
Computed tomographic studies were performed on a 64-slice MDCT (Lightspeed VCT; GE Healthcare, Milwaukee, WI, USA). The heart rate during CT acquisition ranged from 50-97 bpm (mean, 69 bpm). The patients did not receive additional premedications, such as β-blockers, for control of their heart rate.
The MDCT protocol was as follows: slice collimation (64×0.625 mm), gantry rotation time (350 ms), table feed (6 mm/s), tube voltage (120 kVp), and tube current (600 mAs). Eighty mL of contrast agent (Iopromide, Ultravist 300; Schering, Berlin, Germany) was injected intravenously at 5 mL/s for 16 seconds. Fifty mL of saline solution chaser at 5 mL/s for 10 seconds was also injected. All examinations were performed using retrospective electrocardiography (ECG)-gating. Image data was reconstructed using the cardiac image reconstruction algorithm provided with the scanner. Images were reconstructed at consecutive 10% increments of the relative risk (R-R) interval, yielding 10 phases of information. All post-processing was performed on a GE AW Workstation (Advantage Windows Workstation 4.3), using the Card IQ function software (GE Healthcare). Image data was reconstructed in the vertical and parallel images of the MV. The opening and closing phases of the MV were selected visually for image analysis. Window settings were adjusted to properly visualize the valve with less beam-hardening artifact.
The images were analyzed using an Image-Pro Plus Image analyzer (Media Cybernetics, Bethesda, MD, USA) and the values determined for the geometric orifice area (GOA) and valve length by 64-slice MDCT were compared with the manufacturer's values. The open and closing angles were measured with a protractor. The manufacturer's values were approximately 10° for the open angle and 120-130° for the closing angle (Fig. 1). Figs. 2 and 3 give examples of the morphologic and functional assessment by MDCT after undergoing valve replacement.
Data are expressed as the mean and standard deviation (SD), and statistical analysis was done using Statistical Package for Social Science (SPSS) 13.0 (SPSS Inc., Chicago, IL, USA). Linear regression analysis and the limits of agreement according to Bland and Altman were determined to compare geometric parameters of the bileaflet mitral MV between the manufacturer's values and the MDCT measurements. Student's t-test was used to compare the manufacturer's values with those determined by MDCT. P<0.05 was considered statistically significant.
The mean age of the patients (10 females and 10 males) in the study was 50±12 years. Ten patients received replacement of mitral or aortic bileaflet MVs. Five patients received double MV replacements. The mean follow-up duration after undergoing bileaflet MV replacement was 99±74 months.
The mean size of the bileaflet aortic MVs was 21.5±2.1 mm (range, 19-25 mm) and the bileaflet mitral MVs was 29.3±2.0 mm (range, 25-33 mm). The manufacturer's values and the MDCT-determined GOAs were 3.4±0.3 cm2 and 3.4±0.2cm2 for the mitral valves and 2.1±0.4 cm2 and 2.1±0.3cm2 for the aortic valves, respectively. The correlation coefficients for the GOAs based on the manufacturer's values compared with those determined by MDCT were 0.433 for the mitral valves and 0.874 for the aortic valves (p<0.001) (Fig. 4A).
The Bland-Altman analysis of bias revealed that there was no significant bias between the GOAs based on the manufacturer's values and the MDCT measurements for the mitral valves (observed bias, 1.121; 95% confidence interval, 0.823-1.1418) (Fig. 4B) and for the aortic position valves (observed bias, 0.938; 95% confidence interval, 0.642-1.223; p<0.001) (Fig. 4C).
The manufacturer's values and the MDCT-determined valve lengths were 29.3±1.99 mm and 28.6±1.65 mm for the mitral valves and 21.5±2.1 mm and 20.7±2.3 mm for the aortic valves, respectively. The correlation coefficients between the valve lengths based on the manufacturer's values compared with the MDCT measurements were 0.651 for the mitral valves and 0.846 for the aortic valves (p<0.001) (Fig. 5A).
The Bland-Altman analysis of bias revealed that there was no significant bias between the lengths based on the manufacturer's values and the MDCT measurements for the mitral valves (observed bias, 0.791; 95% confidence interval, 0.639-0.934) (Fig. 5B) and for the aortic valves (observed bias, 1008; 95% confidence interval, 0.702-1.313; p<0.001) (Fig. 5C). The opening and closing angles determined by MDCT were 10.9±0.6° and 131.1±3.2° for the mitral valves and 11.1±0.9° and 120.6±1.7° for the aortic valves, respectively.
The use of MDCT has facilitated the non-invasive detection of coronary artery calcifications,13) visualization of the lumens and walls of the coronary arteries, and the ability to obtain information on the presence and severity of coronary artery disease (CAD).9-12) Over the last several years, a dramatic improvement in MDCT technology has allowed for an assessment of valve morphology and calcification in patients with mitral and aortic stenoses.21)22)
MDCT has led to advances in the assessment of cardiovascular anatomy and function and has created new clinical applications in cardiovascular imaging.23) These applications include follow-up of percutaneous coronary intervention,15)16) follow-up of coronary artery bypass grafts,17-20) assessment of the anatomy of the pulmonary vein of patients with atrial fibrillation,24-28) and determination of the coronary sinus of patients planning cardiac resynchronization therapy. Some studies have reported that the MDCT is a sensitive and objective method for accessing the morphology and calcification of native aortic and mitral valves.21)22)
TTE is recommended in a step-by-step approach in the evaluation of patients with suspected prosthetic valve dysfunction. If a high gradient is detected, additional tests may be needed, including TEE and a fluoroscopic examination. However, TTE has some limitations (i.e., excessive metallic artifacts), and TEE and fluoroscopic examination do not always provide for a definitive diagnosis.
For both the GOAs and valve lengths, the values determined by 64-slice MDCT were as accurate as the manufacturer's values based on correlation coefficients, not only for the mitral valves (GOA, 0.433; length, 0.651; p<0.001), but also for the aortic valves (GOA, 0.874; length, 0.846; p<0.001). Therefore, we consider 64-slice MDCT to be an accurate modality for the morphologic and functional assessment of bileaflet MVs.
It is well-known that 64-slice MDCT requires exposure to a higher radiation dose {approximately 10 milli-Sieverts (mSv)} than the mean effective dose of diagnostic coronary artery angiography (usually between 3.0 and 6.0 mSv). To evaluate and generalize the function and morphology of bileaflet MVs using MDCT, patient selection should made with care.
The first limitation of this study was it was only performed on patients who received SJM valves, a kind of bileaflet MV and the majority of patients had normal left ventricular function. Our study did not include patients with Ball-in-cage type valves (e.g., the Starr-Edwards prostheses) or other bileaflet mechanical prostheses (e.g., the Carbomedics prostheses and bioprostheses).
Secondly, it may be questioned whether an assessment of the GOA or length by 64-slice MDCT is superior for hemodynamic conditions, such as significant regurgitation. Although panni, thrombi, and vegetations were not detected in this study, the opening and closing angles determined by 64-slice MDCT may also provide better information for a severe mismatching valve. This will require future study.
MDCT is a powerful and promising modality by which to assess the function and morphology of bileaflet MVs. If appropriate patient selection has been undertaken, such as for patients with a normal sinus rhythm, a poor echo window on TTE, refusal to undergo TEE, and patients in a clinically stable condition, the optimal use of this technology could prove essential for comprehensive evaluation of valve function.
Figures and Tables
Fig. 1
The GOAs, lengths, and opening/closing angles of SJM valves determined by 64-slice MDCT. The images are analyzed using an Image-Pro Plus Image module (Media Cybernetics) and were compared with the manufacturer's values for the GOAs, lengths (A), opening angles (B), and closing angles (C) of the SJM valves as determined by 64-slice MDCT. GOAs and lengths were measured on the vertical image of the mechanical valve. The opening and closing angles were measured on parallel images of the mechanical valve. GOA: geometric orifice area, SJM: St. Jude Medical, MDCT: multidetector computed tomography.
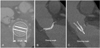
Fig. 2
A 39-year-old woman who presented for assessment of function after undergoing SJM mitral valve replacement (valve size, 29 mm) for infective endocarditis. In the mitral valve, the GOA determined by MDCT was 3.35 cm2 and the manufacturer's value was 3.5 cm2. The valve length determined by MDCT was 28.6 mm and the manufacturer's value was 29 mm. The opening angle determined by MDCT was 11.2° and the manufacturer's value was 10°, and the closing angle determined by MDCT was 132.1° and the manufacturer's value was 130°. A: a vertical reformatted image of the valve shows the SJM mitral valve (#29) with symmetric opening of mechanical components. The valve is intact, based on measurement of the GOA and the length. B: a parallel reformatted image of valve shows the SJM mitral valve (#29). The valve is intact based on measurement of the opening and closing (not shown) angles. SJM: St. Jude Medical, GOA: geometric orifice area, MDCT: multidetector computed tomography.
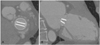
Fig. 3
A 54-year-old woman who presented for assessment of function after undergoing SJM aortic valve replacement (valve size, 19 mm) for severe aortic stenosis. In the aortic valve, the GOA determined by MDCT was 1.68 cm2 and the manufacturer's value was 1.7 cm2. The valve length determined by MDCT was 18.6 mm and the manufacturer's value was 19 mm. The opening angle determined by 64-slice MDCT was 11.7° and the manufacturer's value was 10°, and the closing angle determined by MDCT was 119.6° and the manufacturer's value was 120°. A: a vertical reformatted image of valve shows the SJM aortic valve (#29) with symmetric opening of the mechanical components. The valve is intact based on measurement of the GOA and the length. B: a parallel reformatted image of valve shows the SJM aortic valve (#29) with symmetric opening of the mechanical components. The valve is intact based on measurement of the opening (not shown) and closing angles. SJM: St. Jude Medical, GOA: geometric orifice area, MDCT: multidetector computed tomography.

Fig. 4
The correlation coefficients and Bland-Altman analysis for the GOA based on the manufacturer's value and determined by 64-slice MDCT. A: the correlation coefficients for the GOA based on the manufacturer's value compared with those determined by MDCT were 0.433 for the mitral valve and 0.874 for the aortic valve (p<0.001). B: the Bland-Altman analysis of bias revealed that there were no significant bias between the GOA based on the manufacturer's value and MDCT for the mitral valve (observed bias, 1.121; 95% confidence interval, 0.823-1.1418). C: the Bland-Altman analysis of bias revealed that there were no significant bias between the GOA based on the manufacturer's value and MDCT for the aortic valve (observed bias, 0.938; 95% confidence interval, 0.642-1.223; p<0.001). The solid line is the mean difference; the dotted lines mark the standard deviations of the differences. Mean GOA=(manufacturer's value+MDCT)/2. GOA: geometric orifice area, MDCT: multidetector computed tomography.

Fig. 5
The correlation coefficients and Bland-Altman analysis for the valve length based on the manufacturer's value and determined by 64-slice MDCT. A: the correlation coefficients for the valve lengths based on the manufacturer's values compared with those determined by MDCT were 1.145 for the mitral valve and 0.790 for the aortic valve (p<0.001). B: the Bland-Altman analysis of bias revealed that there was no significant bias between the GOA based on the manufacturer's value and MDCT for the mitral valve (observed bias, 1.121; 95% confidence interval, 0.823-1.1418). C: the Bland-Altman analysis of bias revealed that there was no significant bias between the GOA based on the manufacturer's value and MDCT for the aortic valve (observed bias, 0.938; 95% confidence interval, 0.642-1.223; p<0.001). The solid line is the mean difference; the dotted lines mark the standard deviations of the differences. Mean length GOA=(manufacturer's value+MDCT)/2. GOA: geometric orifice area, MDCT: multidetector computed tomography.

Acknowledgments
This study was supported by a grant of the Seoul R & BD Program of the Republic of Korea (#10526).
References
1. Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002. 15:167–184.
2. Chambers J, Deverall P. Limitations and pitfalls in the assessment of prosthetic valves with Doppler ultrasonography. J Thorac Cardiovasc Surg. 1992. 104:495–501.
3. Muratori M, Montorsi P, Teruzzi G, et al. Feasibility and diagnostic accuracy of quantitative assessment of mechanical prostheses leaflet motion by transthoracic and transesophageal echocardiography in suspected prosthetic valve dysfunction. Am J Cardiol. 2006. 97:94–100.
4. Montorsi P, De Bernardi F, Muratori M, Cavoretto D, Pepi M. Role of cine-fluoroscopy, transthoracic, and transesophageal echocardiography in patients with suspected prosthetic heart valve thrombosis. Am J Cardiol. 2000. 85:58–64.
5. Koo SH, Kim SH, Oh SI, et al. Echocardiographic characteristics of normally functioning CarboMedics and St. Jude medical mitral valve. Korean Circ J. 1995. 25:469–476.
6. Koo SH, Sung JD, Park SS, et al. Clinical utility of transesophageal echocardiography (TEE) in prosthetic valve dysfunction. Korean Circ J. 1993. 23:928–938.
7. Kim YN, Song YS, Kim KS, Kim KB, Huh SH, Choi SY. Evaluation of functional regurgitation flow in patients with clinically normal mitral prosthesis by transesophageal echocardiography. Korean Circ J. 1993. 23:67–74.
8. Joo SJ, Hyon MS, Doh MH, et al. Changes of Doppler echocardiographic findings after mitral valve operation. Korean Circ J. 1987. 17:649–660.
9. Achenbach S, Ulzheimer S, Baum U, et al. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation. 2000. 102:2823–2828.
10. Nieman K, Oudkerk M, Rensing BJ, et al. Coronary angiography with multi-slice computed tomography. Lancet. 2001. 357:599–603.
11. Achenbach S, Giesler T, Ropers D, et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically-gated, multislice spiral computed tomography. Circulation. 2001. 103:2535–2538.
12. Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005. 112:2318–2323.
13. Becker CR, Kleffel T, Crispin A, et al. Coronary artery calcium measurement: agreement of multirow detector and electron beam CT. AJR Am J Roentgenol. 2001. 176:1295–1298.
14. Schuijf JD, Bax JJ, Salm LP, et al. Noninvasive coronary imaging and assessment of left ventricular function using 16-slice computed tomography. Am J Cardiol. 2005. 95:571–574.
15. Groen JM, Greuter MJ, van Ooijen PM, Oudkerk M. A new approach to the assessment of lumen visibility of coronary artery stent at various heart rates using 64-slice MDCT. Eur Radiol. 2007. 17:1879–1884.
16. Rixe J, Achenbach S, Ropers D, et al. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J. 2006. 27:2567–2572.
17. Muhlenbruch G, Mahnken AH, Das M, et al. Evaluation of aortocoronary bypass stents with cardiac MDCT compared with conventional catheter angiography. AJR Am J Roentgenol. 2007. 188:361–369.
18. Jones CM, Athanasiou T, Dunne N, et al. Multi-detector computed tomography in coronary artery bypass graft assessment: a meta-analysis. Ann Thorac Surg. 2007. 83:341–348.
19. Ropers D, Pohle FK, Kuettner A, et al. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation. 2006. 114:2334–2341.
20. Salm LP, Bax JJ, Jukema JW, et al. Comprehensive assessment of patients after coronary artery bypass grafting by 16-detector-row computed tomography. Am Heart J. 2005. 150:775–781.
21. Hoffmann U, Ferencik M, Cury RC, Pena AJ. Coronary CT angiography. J Nucl Med. 2006. 47:797–806.
22. Dawson P. Multi-slice CT contrast enhancement regimens. Clin Radiol. 2004. 59:1051–1060.
23. Willmann JK, Kobza R, Roos JE, et al. ECG-gated multi-detector row CT for assessment of mitral valve disease: initial experience. Eur Radiol. 2002. 12:2662–2669.
24. Willmann JK, Weishaupt D, Lachat M, et al. Electrocardiographically gated multi-detector row CT for assessment of valveular morphology and calcification in aortic stenosis. Radiology. 2002. 225:120–128.
25. Budoff MJ, Cohen MC, Garcia MJ, et al. ACCF/AHA clinical competence statement on cardiac imaging with computed tomography and magnetic resonance. Circulation. 2005. 112:598–617.
26. Chiang SJ, Tsao HM, Wu MH, et al. Anatomic characteristics of the left atrial isthmus in patients with atrial fibrillation: lessons from computed tomographic images. J Cardiovasc Electrophysiol. 2006. 17:1274–1278.
27. Cury RC, Abbara S, Schmidt S, et al. Relationship of the esophagus and aorta to the left atrium and pulmonary veins: implications for catheter ablation of atrial fibrillation. Heart Rhythm. 2005. 2:1317–1323.
28. Schwartzman D, Lacomis J, Wigginton WG. Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J Am Coll Cardiol. 2003. 41:1349–1357.
29. Hunold P, Vogt FM, Schmermund A, et al. Radiation exposure during cardiac CT: effective doses at multi-detector row CT and electron-beam CT. Radiology. 2003. 226:145–152.
30. Morin RL, Gerber TC, McCollough CH. Radiation dose in computed tomography of the heart. Circulation. 2003. 107:917–922.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download