Abstract
Massive deposits of fat around heart are seen in overweight persons and are associated with coronary artery disease. Investigators have focused on the clinical significance of epicardial fat with respect to metabolic effects such as insulin resistance and inflammation, but the mechanical effects, such as constriction, have been largely ignored. We present an unusual case of a 59-year-old woman with obesity and diabetes mellitus who had been undergoing peritoneal dialysis due to end-stage renal disease, and who developed constrictive pericarditis, possibly secondary to extensive epicardial fatty accumulation.
Human epicardial fat is visceral thoracic fat because of its apposition to the heart. Like other white adipose tissue loci, such as the abdominal adipose tissue, epicardial fat might function as a lipid storing depot, as an endocrine organ secreting hormones, and as inflammatory tissue secreting cytokines and chemokines.1) Recently, it was reported that epicardial adipose tissue measured by transthoracic echocardiography in obese subjects was well correlated with abdominal visceral adipose tissue assessed by MRI2) and that echocardiographic measurement of epicardial fat might provide additional information in the risk assessment of coronary artery disease and prediction of coronary artery disease extent and activity.3) However, extensive epicardial fatty accumulation has never been reported to exert a constrictive effect on the heart.
Constrictive pericarditis is associated with the healing of acute pericarditis or chronic pericardial effusion, both of which are followed by firm scar formation, fibrosis of the pericardium, and impaired ventricular filling In Korea, a large number of pericardial effusion and constrictive pericarditis cases have been associated with tuberculosis.4)5) However, chronic constrictive pericarditis can also be a sequela to trauma, cardiac surgery, irradiation, infection, connective tissue disease, and chronic kidney disease with uremia treated by dialysis.
We present the first case of constrictive pericarditis caused by massive epicardial fatty accumulation. A 59-year-old woman with end-stage renal disease presented with dyspnea and peripheral edema in spite of undergoing additional dialysis to control uremia and hypervolemia during a period of two weeks. The patient was examined using transthoracic echocardiogram (TTE), computed tomography (CT), and cardiac catheterization.
A 59-year-old woman was admitted to the hospital because of progressive dyspnea, peripheral edema, and general weakness. She had a six-year history of end-stage renal disease and peritoneal dialysis. She also had a history of diabetes mellitus, hypertension, and parathyroidectomy due to parathyroid adenoma. She denied alcohol use and corticosteroid use and had no history of tuberculous infection or tuberous sclerosis.
She was 156 cm tall and weighed 72 kg, with a body mass index of 29.6 kg/m2. Her blood pressure was 90/60 mmHg, and her pulse rate was 95 beats/min. Facial edema and neck vein distension were noted on physical examination. No palpable masses were found in the neck, trunk, or axillae. Respiratory examination revealed clear breathing sound bilaterally, with no crackles or wheezes. Heart sounds were decreased, but no murmur or pericardial rub was heard. Pitting edema was noted in both legs. The electrocardiogram was within normal limits. Laboratory examination showed evidence of end-stage renal disease, including blood urea nitrogen of 50.8 mg/dL and creatinine of 9.13 mg/dL. The other laboratory parameters, including autoantibodies, were unremarkable.
Chest radiography revealed cardiomegaly and bilateral obliteration of the costophrenic angles, indicating the presence of a pleural effusion (Fig. 1). TTE showed a small pericardial effusion and a diffuse, circumferential echogenic mass in the epicardium, suggesting massive fat infiltration. There were no significant abnormalities in systolic or diastolic function. The left ventricular (LV) ejection fraction was estimated to be 68% (Fig. 2). There was no evidence of right ventricular (RV) dysfunction, based on fractional area change.
The patient underwent high concentrative peritoneal dialysis and additional hemodialysis to control uremia and hypervolemia. Despite management over a two-week period, her symptoms and signs of hemodynamic instability failed to improve. Follow-up TTE revealed no residual pericardial effusion, but continued to show diffuse, massive fat infiltration. Moreover, the patient had pericardial thickening and adhesions, interventricular septum bouncing, inferior vena cava plethora, diastolic reversal of hepatic venous flow, and respiratory variations on transmitral inflow (Fig. 3), suggesting the presence of constrictive pericarditis. On tissue Doppler imaging, the average of the pulsed Doppler-derived E' velocity at the septal corner was 5 m/s (Fig. 3F). Multislice CT of the heart confirmed thickened pericardium (7-11 mm) and diffuse, extensive fat infiltration of the whole pericardium, with a density similar to that of fat (Fig. 4). We measured the volume of the epicardial adipose tissue using 3-mm thick axial slices. We manually traced the epicardial fat in every slice, working from the aortic root to the apex. The number of manually traced slices was 37. The measured volume of epicardial fat in this patient was 487 mL.
Coronary angiography and left ventriculogram showed minimal intracoronary stenosis and normal systolic function, but also showed decreased normal inward systolic motion of the coronary arteries, caused by interference from massive surrounding fat tissues. Cardiac catheterization showed diastolic pressure equalization, "dip and plateau" feature on simultaneous right ventricular and left ventricular pressure tracings (Fig. 5A), equalization of right atrium and left ventricular pressures on simultaneous right atrial and left ventricular pressure tracings, and marked x & y descent on right atrial pressure tracings (Fig. 5B). These findings were compatible with constrictive pericarditis.
We therefore diagnosed this patient with constrictive pericarditis that may have been promoted by massive epicardial fat. We prepared the patient for pericardiectomy to improve hemodynamics, after consultation with the department of cardiac surgery. The patient developed sudden exacerbation of hemodynamic instability during preparation. Pericardiectomy could not be performed, and the patient expired.
Human epicardial adipose tissue is a visceral fat that secretes proatherogenic, proinflammatory, and prothrombotic hormones, cytokines, and chemokines.6) Epicardial adipose tissue is also implicated in the pathogenesis of coronary atherosclerosis because of its proximity to the adventitia of the coronary arteries.7) Because there is no fibrous layer to interfere with the diffusion of adipokines and free fatty acids, epicardial fat could influence coronary atherosclerosis and myocardial function.1) Epicardial fat thickness, measured by echocardiography, is increased in obesity and metabolic syndrome, and this may provide additional information for predicting the extent and activity of coronary artery disease.3) In some patients, a marked increase in epicardial fat may impair myocardial relaxation and alter ventricular diastolic filling.8) However, no investigator has ever reported that epicardial fat can have a constrictive effect on the heart secondary to its massive volume.
In the current case, massive epicardial fatty accumulation and pericardial effusion were noted at initial TTE. Despite management over a two-week period, pericardial thickening and constrictive physiology had developed by the time of the follow-up echocardiogram. We were not able to completely assess the cause of pericarditis in this patient because she expired before pericardiectomy was performed. Uremia might have been the cause of pericarditis because there was no evidence of irradiation, trauma, tuberculosis, or connective tissue disorder, but there was a history of end-stage renal disease being treated with dialysis. The chance of developing constrictive pericarditis following a bout of acute pericarditis is unknown, but it is undoubtedly extremely low.9)
The measurement of epicardial fat volume is feasible using multislice CT. In a study looking at the measurement of epicardial fat volume using multislice CT in a series of 151 adults, epicardial fat volume ranged from 25 mL to 274 mL.10) In the current case, the epicardial volume was 487 mL, which corresponded to a fat mass of 448 g using a conversion factor of 0.92 (specific weight of fat). Because of the abnormally increased mass of epicardial fat, we initially thought of abnormal pathology, such as cardiac lipomatosis. For the diagnosis of cardiac lipomatosis, biopsy was required to confirm true encapsulated neoplasms composed of mature adipose tissue.11) Although we could not perform a biopsy, epicardial fat rather than cardiac lipomatosis was the proper diagnosis, because the adipose tissues were distributed diffusely rather than scattered around the heart, and because there was no evidence of encapsulating mass on TTE and CT.11) In our opinion, the mechanical effect of the massive epicardial fat played a major role in the clinical manifestations, even though there was minimal pericardial change. Hence, it is conceivable that the unwanted interaction between space-occupying, massive epicardial fat and pericardial thickening might restrict the diastolic motion of the heart and induce constrictive physiology on TTE.
The diagnosis of constrictive pericarditis remains difficult to make. Two-dimensional and Doppler echocardiography, CT or MRI of the pericardium, and conventional cardiac catheterization are useful in the diagnosis.9) Two-dimensional echocardiography findings include pericardial thickening, abrupt displacement of the interventricular septum during early diastole (septal "bounce"), dilation of the hepatic veins, and plethora of the inferior vena cava. Doppler flow velocity measurements reveal exaggerated respiratory variation in mitral inflow velocity-greater than or equal to a 25% increase in mitral early inflow (E) velocity-and increased diastolic flow reversal in the hepatic veins on expiration. In the current case, two-dimensional echocardiography and Doppler revealed these findings, suggesting the presence of constrictive pericarditis. One study using tissue Doppler echocardiography suggested that an E' cut-off value ≥8 cm/s could distinguish constrictive pericarditis from restrictive cardiomyopathy.12) However, another study showed attenuated pulsed-wave Doppler E' velocity (<8 cm/s) in 3 of 11 constrictive pericarditis patients.13) In our case, the average pulsed Dopplerderived E' velocity at the septal corner was 5 cm/s. Although the E' velocity was attenuated below 8 cm/s, constrictive pericarditis could not be ruled out.
Demonstration of a thickened pericardium using CT is helpful in the diagnosis of constrictive pericarditis. The thickness of the normal pericardium is less than 2 mm.14) In our case, CT demonstrated a thickened pericardium (7-11 mm) without calcification, indicating the presence of acute pericarditis. On cardiac catheterization in patients with constrictive pericarditis, LV pressure tracing shows a brief, rapid fall in early diastole, followed by a high early diastolic pressure plateau ("dip-plateau" or "square-root" sign). Right atrial pressure tracing shows a preserved x descent and a prominent y descent. Right atrial, RV diastolic, and pre-a wave LV diastolic pressures show equalization. In our case, all of these signs were observed. Recent study has shown that the ratio of RV to LV systolic area during inspiration and expiration is a reliable catheterization criterion for differentiating constriction from restriction.15) In this case, we did not measure the full LV tracing curves and could not measure the ratio of RV to LV systolic area during inspiration and expiration, because this concept had not been reported at that time. In Fig. 5, the +dP/dt of the RV was significantly decreased. Although this finding suggested RV dysfunction, the possibility remained that surrounding massive epicardial fat attached to the pericardium could interfere with the systolic motion of the relatively thin-walled RV.
In conclusion, epicardial fat can have a mechanical influence on the heart, especially in cases of huge epicardial fat volume combined with pathologic conditions such as pericardial thickening. Constrictive pericarditis associated with huge epicardial fat volume has not been described previously in the literature. The current case broadens the spectrum of potential presentation of epicardial fat.
Figures and Tables
Fig. 1
Chest radiography showed an enlarged cardiac silhouette and obliteration of both costophrenic angles indicating the presence of a pleural effusion.
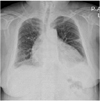
Fig. 2
Initial two-dimensional transthoracic echocardiogram in parasternal view (A) and apical four chamber view (B) showed a small pericardial effusion (arrow), a diffuse and circumferential echogenic mass in the epicardium (arrowhead). Transmitral pulsed-wave Doppler signals (C) showed prolongation of the deceleration time and no significant respiratory variations which were not compatible with constrictive pericarditis. Ejection fraction of LV was estimated to be 68%. LV: left ventricle, LA: left atrium.
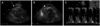
Fig. 3
Follow-up two-dimensional transthoracic echocardiogram showed decreased pericardial effusion, pericardial thickening and adhesion (arrow) (A and B), and inferior vena cava plethora (C). Doppler showed respiratory variations in the transmitral inflow (D) and hepatic vein reversal (E). On tissue Doppler imaging, the average pulsed Doppler-derived E' velocity at the septal corner was 5 m/s (F). The respirometer signal was very poor, and this might have been caused by obesity. I: inspiration, E: expiration.
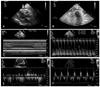
Fig. 4
Multislice computed tomography of the heart showed thickened pericardium and diffuse, extensive fat infiltration of the whole pericardium, with a density similar to that of fat (approximately -40 Hounsfield units) (*). A: oblique sagittal section. B: oblique coronal section. C: axial section.

Fig. 5
Cardiac catheterization. A: cardiac catheterization on simultaneous RV and LV pressure tracings showed equalization of diastolic pressure, as well as a "dip and plateau" feature. B: cardiac catheterization on simultaneous RA and LV pressure tracings showed equalization of RA and LV pressures and marked x & y descent on RA pressure. LV: left ventricle, RA: right atrium, RV: right ventricle.
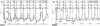
References
1. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007. 153:907–917.
2. Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003. 88:5163–5168.
3. Ahn SG, Lim HS, Joe DY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008. 94:e7.
4. Yang HS, Song JK, Song JM, et al. Clinical characteristics of constrictive pericarditis diagnosed by echo-Doppler technique in Korea. J Korean Med Sci. 2001. 16:558–566.
5. Park SY. The usefulness of pericardial biopsy to evaluate the causes of pericardial disease. Korean Circ J. 1999. 29:517–522.
6. Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007. 101:27–39.
7. Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003. 108:2460–2466.
8. Iacobellis G, Leonetti F, Singh N, M Sharma A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007. 115:272–273.
9. LeWinter MM. Libby P, Bonow PL, Mann DL, Zipes DP, editors. Pericardial disease. Braunwald's Heart Disease: A Text Book of Cardiovascular Medicine. 2008. 8th ed. Philadelphia: Saunders Elsevier;1833.
10. Sarin S, Wenger C, Marwaha A, et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol. 2008. 102:767–771.
11. Salanitri JC, Pereles FS. Cardiac lipoma and lipomatous hypertrophy of the interatrial septum: cardiac magnetic resonance imaging findings. J Comput Assist Tomogr. 2004. 28:852–856.
12. Ha JW, Ommen SR, Tajik AJ, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy using mitral annular velocity by tissue Doppler echocardiography. Am J Cardiol. 2004. 94:316–319.
13. Sengupta PP, Krishnamoorthy VK, Abhayaratna WP, et al. Comparison of usefulness of tissue Doppler imaging versus brain natriuretic peptide for differentiation of constrictive pericardial disease from restrictive cardiomyopathy. Am J Cardiol. 2008. 102:357–362.
14. Oyama N, Oyama N, Komuro K, Nambu T, Manning WJ, Miyasaka K. Computed tomography and magnetic resonance imaging of the pericardium: anatomy and pathology. Magn Reson Med Sci. 2004. 3:145–152.
15. Talreja DR, Nishimura RA, Oh JK, Holmes DR. Constrictive pericarditis in the modern era: novel criteria for diagnosis in the cardiac catheterization laboratory. J Am Coll Cardiol. 2008. 51:315–319.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download