Abstract
Background and Objectives
Rapamycin has been shown to inhibit the vascular smooth muscle cell migration and proliferation that contributes to neointimal formation. We investigated whether the perivascular delivery of rapamycin in Pluronic gel could inhibit neointimal hyperplasia in a rat carotid artery model, and we tested the usefulness of carotid arteriography.
Materials and Methods
To assess the kinetics of rapamycin's release from Pluronic gel, a [3H] thymidine incorporation assay was performed with using the media exposed to rapamycin in Pluronic gel for 10, 20, 60 and 120 min. We applied 100 µg of rapamycin in Pluronic gel to the perivascular space of the carotid artery after the balloon injury (n=9), whereas only gel was applied in a control group (n=9). We performed the carotid arteriography and the morphometric analysis 14 days after injury.
Results
The [3H] thymidine incorporation assay showed a reduction of uptake in a time-dependent manner (86%, 48%, 45% and 40% of the control, respectively, at 10, 20, 60 and 120 minutes). The inhibiting effect of rapamycin on neointimal hyperplasia was identified on the carotid arteriography (mean luminal diameter; 0.75±0.11 vs. 0.60±0.12 arbitrary units, respectively, p<0.05) and on the morphometric analysis (neointima area: 0.09±0.03 vs. 0.17±0.06 mm2, respectively, p<0.05).
Conclusion
This study demonstrated that perivascular delivery of rapamycin in Pluronic gel inhibits neointimal hyperplasia in a rat carotid injury model. This animal model combined with arteriography can be used for developing new drugs to treat restenosis. In addition, this technique might be useful for vascular surgery such as coronary artery bypass grafting, arteriovenous fistula formation and peripheral vascular bypass graft insertion.
Atherosclerosis is one of the major causes of heart attack, stroke and gangrene of the extremities, and it is associated with significant morbidity and mortality. Atherosclerotic lesions are caused by an excessive, inflammatory fibroproliferative response to a variety of insults to the endothelium and smooth muscle of the arterial wall.1) A number of growth factors, cytokines and vasoregulatory molecules participate in this process.1-4) Percutaneous transluminal coronary angioplasty (PTCA) has been useful for performing coronary revascularization. However, its usefulness has been limited by a 30-50 percent restenosis rate within 6 months after the procedure.5)
Vascular injury after angioplasty triggers a vasculoproliferative cascade leading to restenosis: 1) platelet activation and thrombus formation, 2) vascular smooth muscle cell (VSMC) migration, and 3) the proliferation of VSMCs.5)6) The elastic recoil of the arterial wall (negative remodeling) also plays a role in luminal narrowing.6)7) The development of stent technology has helped to overcome the elastic recoil and negative arterial remodeling. However, there continues to be a 15% rate of restenosis in "ideal" lesions and a rate of 60% in "complex" lesions.
The principal pathophysiology of in-stent restenosis is neointimal hyperplasia that's due to VSMC proliferation. 5)7)8) There have been many attempts to prevent restenosis, including atherectomy, brachytherapy, gene therapy and a variety of pharmaceutical agents.8)9) Pharmaceutical agents such as paclitaxel and rapamycin have been applied in animal models and in the clinical setting with systemic dosing and local application with using a drug-delivery catheter, drug-eluting stent or by perivascular delivery.6)10-20)
Rapamycin is a macrocyclic, lypophilic lactone with immunosuppressive and antifungal activity, and this drug is derived from the actinomycete Streptomyces hygroscopicus. Rapamycin is well known to restrict the proliferation of VSMCs by blocking cell-cycle progression at the G1-S cell cycle transition and it also inhibits the migration of VSMCs into the areas of vascular injury.14-16)21) The rapamycin-eluting stent has been reported to reduce the incidence of restenosis and the late loss of the arterial luminal diameter in human clinical trials, as well as in a porcine model.8)16)18-20) Yet stents are a very attractive mode of delivery, but implantation of these devices is not possible in all cases. Perivascular delivery of rapamycin represents a potential alternative to the endoluminal approach. An advantage of perivascular delivery is that the formulation is not in direct contact with blood, thus reducing the risk of thrombosis, and this style of delivery can be useful for vascular surgery. However, the efficacy of a drug applied to the outer surface of a blood vessel on a process taking place in the lumen should be demonstrated.
We investigated whether the perivascular delivery of rapamycin in Pluronic gel could inhibit neointimal hyperplasia after carotid artery injury in a rat model. In addition, we tested the usefulness of carotid arteriography for evaluation of the injury.
Preparation of a 30% F-127 Pluronic gel solution (wt/vol) was carried out and the solution was kept at 4℃ for 24 hours. Next, 1 mg of rapamycin was dissolved in 100 µL of 100% ethanol and 100 µL of this ethanol solution was added to 900 µL of the previously cooled 30% Pluronic gel solution, thus creating 100 µL aliquots of this solution that contained 100 µg of rapamycin.
Twelve to fourteen week old male Sprague-Dawley rats (SD, Samtako, Korea) weighing around 280 g each were fed a normal chow diet and water was given ad libitum. The Chungbuk National University Animal Care and Use Committee approved all the protocols used for the experiments. The animals were anesthetized using an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (6.7 mg/kg). The right common carotid artery was surgically exposed. A 2F Fogarty balloon embolectomy catheter (Baxter, McGraw Park, IL, USA) was advanced along the length of the right common carotid artery and then it was retracted three times with mild balloon inflation pressure. The animals were divided into two groups: 9 rats were in the control group (gel only) and 9 rats were in the rapamycin treatment group. Immediately after the balloon injury, 100 µL of Pluronic gel containing 100 µg of rapamycin was applied to the exposed anterior side of the perivascular space of about 10 mm segments of the injured carotid artery, from 5 mm proximal to the bifurcation of the internal and external carotid arteries to approximately the clavicle line. For the control animals, 100 µL of Pluronic gel was applied to the same area.
Carotid arteriography was performed 14 days after the balloon injury.10) A 4F vascular cannula (Cook, USA) was introduced through the abdominal aorta and this was advanced to the thoracic aorta. Carotid cineangiography was performed by injecting a contrast agent (VisipaqueTM, Amersham health, Cork, Ireland). The angiographic mean luminal diameter (MLD) was measured for the injured segments by performing cumulative analysis with using computerized coronary angiography (Videodensitometry, Phillips, The Netherlands). The mean luminal diameter was described as an arbitrary unit (AU) relative to the contralateral uninjured carotid arterial diameter, which was set at 1 AU.
Following the angiography, the rats were sacrificed and morphometric analysis was then performed. The carotid arteries were perfusion-fixed with 10% buffered formalin. Three individual sections (5 µm) from the middle of each artery with Pluronic gel were stained with hematoxylin-eosin and these were used for morphometric analysis by an investigator who was kept "blind" to the experimental procedure. The cross-sectional area (Aintima and Amedia), the area ratio (Aintima/Amedia) and the percent area stenosis (% stenosis), indicating the proportion of neointima to the lumen, were measured by the Image Inside computer program (version 2002, Focus, Korea).
The effect of rapamycin versus vehicle on in situ VSMC proliferation was measured by 5-bromo-2-deoxyuridine (BrDU, Sigma Chemical Company, USA) incorporation at 48 hours after injury.24) Briefly, the rapamycin-treated rats and vehicle-treated rats (each group n=4) were injected subcutaneously with BrDU (30 mg/kg) at 30, 38 and 46 hours after injury. The carotid artery sections were harvested at 48 hours after injury and the histological sections were incubated with mouse anti-BrDU monoclonal antibodies (Boehringer Mannheim, Germany). The mitotic index (%) was described as the fraction of BrDU-positive nuclei in the medial VSMCs per cross section.
The effect of rapamycin on VSMC proliferation in vitro and the release of rapamycin from the Pluronic gel were assessed by a [3H] thymidine incorporation assay.27) The VSMCs were prepared by enzymatic digestion of the aortas from the SD rats with using collagenase type I (167.5 U/mL), elastase type III (15 U/mL) soybean trypsin inhibitor (364 g/mL) and bovine serum albumin (2 mg/mL). Rat VSMCs (passages 6-8) were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Gibco-BRL, USA) supplemented with 10% fetal bovine serum (FBS) in a CO2 incubator (5% CO2, 37℃). The cells were plated (1×103 cells per well) on 24-well plates at ~70% confluence. They were incubated for 24 hours with 1 mL of serum-free media. Thereafter they were incubated with six different serum-free media as follows for another 24 hours: media exposed to the control-gel for 120 minutes, media with rapamycin-10 µM (9.14 µg/mL), and four types of media exposed to rapamycin in the Pluronic gel for 10, 20, 60 and 120 minutes, respectively (Fig. 1). At 20 hours after changing the media to DMEM and 5% FBS, each culture was pulsed with 1 µCi [3H] thymidine (Amersham, USA) and this was incubated for 4 hours. At the end of incubation, the cells were washed sequentially with ice-cold phosphate-buffered saline (PBS), 10% trichloroacetic acid (TCA) buffer and a mixed solution of ethanol with ether, and then the cells were solubilized in 0.5 n NaOH. The thymidine uptake (cpm) was measured by a liquid scintillation counter.
The carotid arteriography in the control group showed distinct narrowing of the injured right carotid artery (Fig. 2A). By contrast, luminal narrowing was not marked in the rapamycin group (Fig. 2B, double arrow). The MLD of the injured carotid artery was compared with that of the uninjured left carotid artery (set at 1 AU). The mean of MLDs in the rapamycin group was significantly larger as compared to that of the control group (0.75±0.11 vs. 0.60±0.12 AU, respectively, n=9, p<0.05, Table 1). There was a narrow segment at the lower region of the right carotid artery, beneath the clavicle in the rapamycin group, which was injured but not treated with rapamycin (Fig. 2B single arrow).
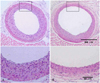
The morphometric analysis showed that the rapamycin group had a significant reduction in neointimal formation compared to the control group (neointima area 0.09± 0.03 vs. 0.17±0.06 mm2, respectively, p<0.05). The neointima/media area ratio (0.77±0.21 vs. 1.45±0.47, respectively, p<0.05) and percent area stenosis (24.0±5.7 vs. 47.6±13.9%, respectively, p<0.05) also showed significant differences in the rapamycin group. However, the changes in the media area (0.12±0.01 vs. 0.11±0.01 mm2, respectively, p>0.05) were not different between the two groups. These results suggested that the medial wall retained its integrity despite the administration of rapamycin (Fig. 3) (Table 1). The histological evaluations in the rapamycin group showed an eccentric pattern of neointimal formation. This was seen primarily at the anterior part of the artery opposite to the location of the posterior neck muscle. However, the control group histology showed involvement of the whole wall.
The effect of rapamycin on in vivo VSMC proliferation was measured by BrdU incorporation at 48 hours after injury. The mitotic index was significantly reduced in the rapamycin group compared to the control group (11.0±7.1% vs. 28.4±5.9%, respectively, p<0.05).
The [3H] thymidine incorporation assay showed a reduction of the uptake in a time-dependent manner (86%, 48%, 45% and 40% of the control, respectively), which indicated that rapamycin was released from the Pluronic gel continually and it inhibited VSMC proliferation (Table 2). The concentration of the released rapamycin from the media that was exposed to rapamycin in the Pluronic gel for 10 min was similar to 10 µM of rapamycin, as determined by the radiouptake (62,780 vs. 59,523 cpm). During 30 minutes of incubation, the release of rapamycin increased rapidly so that the radiouptake showed about half of the amount compared to the control. Thereafter, the radiouptake was slowly reduced.
Rapamycin is a macrolide antibiotic with antifungal and immunosuppressive activities. Rapamycin is known to inhibit the development of arteriopathy in allograft transplantation and it reduces neointimal hyperplasia in arteriovenous grafts and in-stent restenosis.14)19)20)22) Rapamycin has antiproliferative activity in VSMC that is mediated by blocking the G1-S transition of the cell cycle.6)15) Inhibition of VSMC proliferation can be initiated by the binding of rapamycin to its cytosolic receptor, FKBP12. This is associated with reduced cdc2, cdk2 and pRb-retinoblastoma protein-phosphorylation activity and increased levels of cyclin-dependent kinase inhibitor (CDKI), p27kipl.2)15)16) In addition, rapamycin inhibits VSMC migration and inflammation.16)22)23)
The findings from the present study demonstrated that the perivascular delivery of rapamycin in a Pluronic gel could inhibit neointimal hyperplasia after carotid artery injury in a rat model. The morphometric analysis showed a significant reduction in the neointimal area, the neointima/media area ratio and the percent area stenosis in the treated rats. In addition, the carotid arteriography revealed less luminal narrowing in the rapamycin group compared to the control group. All the histology sections in the rapamycin group showed eccentric patterns for the inhibition of neointimal formation, like that at the anterior part of the artery opposite to the location of the posterior neck muscle (Fig. 3). By contrast, the control group histology showed concentric patterns of diffuse neointimal formation. The Pluronic gel was applied directly to the exposed anterior side of the perivascular space, on roughly 30-40 percent of the circumference of the right common carotid artery. In the surgical field, the gel was applied with the rats in the recumbent posture and thereafter the rats lived in a prone posture. There were neck muscles behind the common carotid artery, and so the drug had to be localized to the anterior part of the artery. It may be reasonable to compare the drug-applied quadrant in the rapamycin group with a similar quadrant in the control group. Comparison of the neointimal area of the entire vessel might result in a significant underestimation of the inhibition of neointimal formation in our study. However, we measured the whole lumen because it may be difficult to pinpoint the specific area of drug application. Despite the localized effect, the overall results showed a significant difference in neointimal formation between the two groups (Table 1).
The BrDU incorporation assay was done to identify the effects of rapamycin on VSMC proliferation in vivo. The VSMCs show a high rate of proliferation at the first 48-72 hours after the arterial injury. The BrDU injection was administered 30, 38 and 46 hours after the injury and the carotid artery sections were harvested at 48 hours. On immunohistochemistry tagging with monoclonal antibody to BrDU, the mitotic index (%) was significantly decreased in the rapamycin group. This result demonstrates that rapamycin restricted the VSMC proliferation in vivo.
The [3H] thymidine incorporation assay was performed to determine the effects of rapamycin on VSMC proliferation in vitro and the release of rapamycin from the Pluronic gel. Released rapamycin from the Pluronic gel affected the radioactive uptake (cpm) of [3H] thymidine. Rapamycin plus gel was left in a water bath for 10, 30, 60 and 120 minutes. The Pluronic gel mass containing the rapamycin was continually degraded in the water bath over time. A very small amount of gel was remained on the bottom of the column after 2 hours of incubation (Fig. 1). We hypothesized that if rapamycin was released from the Pluronic gel and it caused inhibition of VSMC proliferation, then the radioactivity identified by [3H] thymidine uptake would decrease. Our study showed that the radioactive uptake using the media exposed to rapamycin in Pluronic gel for 10 minutes was 62,780 cpm/well, which was 82% of that of the control and comparable to the rapamycin-10 µM (Table 2). The uptake using the media exposed for 30 minutes was almost 50% of that of the control. Thereafter the uptake showed a gradual reduction, and it finally reached 40% compared to the control. Thus, these findings suggest that the Pluronic gel effectively released rapamycin and that the pharmacokinetics of the drug worked well through this polymer, though it would have been ideal to measure the concentration of rapamycin in the tissue directly.
Many of the studies performed using local drug delivery have focused on the use of stents.7)16)19)20) Studies on drug-eluting stents can only be done in large animals such as rabbits and pigs.14)16)24) However, local perivascular drug application is advantageous for reducing the cost and time of performing experiments with small animals such as rats.21)22)25)26) Drug-eluting stents are an attractive mode of delivery, but implantation of these devices is not always possible. Perivascular drug delivery can be useful for vascular surgery such as coronary artery bypass grafting, arteriovenous fistula formation and peripheral vascular bypass graft insertion.22)24) Furthermore, injectable for mulations based on gels can be used for injection in the perivascular space via a catheter or needle with using minimally invasive procedures.
The F-127 Pluronic gel was the polymer used in this study, and it has the novel property of being soluble at 4℃ and being solid at 37℃. This characteristic makes pharmaceutical compounding easily because it can be drawn into a syringe for accurate dose measurement at a low temperature. It is also useful for local drug application that is in contact with living tissues at 37℃. There was no gross evidence of inflammation or a foreign body response to either the drug or the vehicle-loaded Pluronic gel. None of the rats died during the study. This results of our study show that the Pluronic gel is useful for local drug delivery in terms of both its safety and the effective release of drug.17)21)
Although angiography is the gold standard for the evaluation of vessel stenosis in the clinical setting, this is very difficult to perform in small animal experiments. We have recently developed carotid arteriography in the rat carotid injury model and we used it for our previous studies (via the oral and endoluminal approach).10)13) At that time we could use only the minimum luminal diameter (one point measurement) for technical reasons. In this experiment, we were able to use the mean luminal diameter and so we systematically evaluated this angiographic method. This measurement was used to evaluate the entire length of the affected artery and to compare the peculiar features of the injury, the drug application and the normal artery on the contralateral side. The uninjured left carotid artery was an internal control. Although morphometric analysis is the standard method used in animal studies, mistakes can occur such as tissue scalloping during the fixation process or not cutting through the horizontal axis perpendicular to the longitudinal arterial axis in the fixed tissue. These problems may have caused under-estimating or over-estimating the findings on the morphometric analysis. Therefore, evaluation of the artery via angiography provides results that can improve the accuracy of the study findings. Although arteriography visualizes the inner luminal diameter of the artery, it can't show the neointimal formation and the presence of negative or positive remodeling.4)27)28) Therefore, the use of both methods complements each other and provides the most accurate results. Fig. 2B illustrates a narrow segment beneath the clavicle, where an injured segment was not exposed to the drug application. This segment provides an interesting comparison of a treated segment and an untreated segment in the same carotid artery, and the findings show the localized and site-specific characteristics of the rapamycin application in the Pluronic gel.
In conclusion, the results of this study demonstrated that perivascular delivery of rapamycin in Pluronic gel inhibits neointimal hyperplasia and it reduces luminal narrowing after injury to the carotid artery in a rat model. The carotid arteriography was complementary to the morphometric analysis; it showed the entire lesion with vascular stenosis without a time delay. This animal model combined with arteriography can be used for the development of new drugs and determining their appropriate doses for the treatment of restenosis.10-12)21)22) In addition, this technique might be useful for vascular surgery such as coronary artery bypass grafting, arteriovenous fistula formation and peripheral vascular bypass graft insertion.22)24)
Figures and Tables
Fig. 1
Degraded gel containing rapamycin according to the incubation time. 500 µL of the Pluronic gel containing 500 µg of rapamycin in 5 mL of serum-free media were applied separately; each column was left in the water bath for 10, 30, 60 and 120 minutes, respectively. The Pluronic gel mass containing rapamycin was continually degraded in the water bath over time. A very small amount of gel was remained on the bottom of the column after 120 minutes of incubation.

Fig. 2
A representative carotid arteriographic finding at 14 days after injury. Diffuse and significant narrowing of the right carotid artery (double arrows) was observed in the control group (A). The rapamycin treated group (B) showed less narrowing of the lumen (double red arrows) compared to the untreated control group. A narrow injured segment (single yellow arrow) was seen beneath the clavicular line where the drug could not be applied.
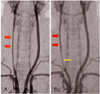
Fig. 3
Representative cross sections (5 µm) of the rat carotid artery at 14 days after balloon injury were stained with hematoxylin-eosin. A: the control group showed a concentric pattern of diffuse neointimal formation. B: rapamycin inhibited the proliferation of vascular smooth muscle cells. Note the eccentric pattern of inhibition resulting from perivascular application of rapamycin in the Pluronic gel at the anterior part of the vessel wall across the posterior neck muscle, over roughly one third of the circumference (upper panels ×100, lower panels ×400).
Acknowledgments
This work was supported by a grant (R01-2006-000-10020-0) of the Basic Research Program of the Korea Science & Engineering Foundation, a grant (0405-ER01-0304-0001) from the Korean Heath 21 R&D Project, Ministry of Health & Welfare, and a research grant from Chungbuk National University in 2006.
References
1. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993. 362:801–809.
2. Chen D, Krasinski K, Sylvester A, Chen J, Nisen PD, Andrés V. Down regulation of cyclin-dependent kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27(KIP1), an inhibitor of neointima formation in the rat carotid artery. J Clin Invest. 1997. 99:2334–2341.
3. Waltenberger J. Modulation of growth factor action: implications for the treatment of cardiovascular diseases. Circulation. 1997. 96:4083–4094.
4. Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation on human coronary arteries. Proc Natl Acad Sci U S A. 1990. 87:4600–4604.
5. Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation. 1996. 94:35–43.
6. Sriram V, Patterson C. Cell cycle in vasculoproliferative diseases: potential interventions and routes of delivery. Circulation. 2001. 103:2414–2419.
7. Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet. 2003. 361:247–249.
8. Pearce BJ, McKinsey JF. Current status of intravascular stents as delivery devices to prevent restenosis. Vasc Endovascular Surg. 2003. 37:231–237.
9. Kataoka T, Honda Y, Bonneau HN, Yock PG, Fitzerald PJ. New catheter-based technology for the treatment of restenosis. J Interv Cardiol. 2002. 15:371–379.
10. Kim DW, Kwon JS, Kim YG, et al. Novel oral formulation of paclitaxel inhibits neointimal hyperplasia in a rat carotid artery injury model. Circulation. 2004. 109:1558–1563.
11. Cho MC, Kwak NJ, Piao H, et al. Effect of paclitaxel local delivery on neointimal formation after endothelial denudation of the rat carotid artery. Korean Cire J. 2000. 30:198–207.
12. Kwon JS, Park SS, Kim YG, et al. Perivascular delivery of paclitaxel with F-127 Pluronic gel inhibits neointimal hyperplasia in a rat carotid artery injury model. Korean Circ J. 2005. 35:221–227.
13. Kim DW, Kim YG, Oh TG, Cho MC, Kim ST. Retrovirus-mediated herpes simplex virus kinase gene therapy for the prevention of stenosis in rat carotid artery injury model. Korean Circ J. 1998. 28:977–989.
14. Cagiannos C, Abul-Khoudoud OR, DeRijk W, et al. Rapamycin-coated expanded polytetrafluoroethylene bypass grafts exhibit decreased anastomotic neointimal hyperplasia in a porcine model. J Vasc Surg. 2005. 42:980–988.
15. Gallo R, Padurean A, Jayaraman T, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999. 99:2164–2170.
16. Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of rapamycin reduces neointimal formation in a porcine coronary model. Circulation. 2001. 104:1188–1193.
17. Pires NMM, van der Hoeven BL, de Vries MR, et al. Local perivascular delivery of anti-restenotic agents from a drug-eluting poly (ε-caprolactone) stent cuff. Biomaterials. 2005. 26:5386–5394.
18. Fukuda D, Sata M, Tanaka K, Nagai R. Potent inhibitory effect of sirolimus on circulating vascular progenitor cells. Circulation. 2005. 111:926–931.
19. Sousa JE, Costa MA, Abizaid A, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2003. 107:24–27.
20. Shafiq N, Malhotra S, Pandhi P, Grover A, Uboweja A. A meta-analysis of clinical trials of paclitaxel- and sirolimus-eluting stents in patients with obstructive coronary artery disease. Br J Clin Pharmacol. 2005. 59:94–101.
21. Signore PE, Machan LS, Jackson JK, et al. Complete inhibition of intimal hyperplasia by perivascular delivery of paclitaxel in balloon-injured rat carotid arteries. J Vasc Interv Radiol. 2001. 12:79–88.
22. Schachner T, Zou Y, Oberhuber A, et al. Local application of rapamycin inhibits neointimal hyperplasia in experimental vein grafts. Ann Thorac Surg. 2004. 77:1580–1585.
23. Hafizi S, Mordi VN, Andersson KM, Chester AH, Yacoub MH. Differential effects of rapamycin, cyclosporine A, and FK506 on human coronary artery smooth muscle cell proliferation and signalling. Vascul Pharmacol. 2004. 41:167–176.
24. Rotmans JI, Pattynama PM, Verhagen HJ, et al. Sirolimus-eluting stents to abolish intimal hyperplasia and improve flow in porcine arteriovenous grafts: a 4-week follow-up study. Circulation. 2005. 111:1537–1542.
25. Hu Y, Zou Y, Dietrich H, Wick G, Xu Q. Inhibition of neointimal hyperplasia of mouse vein grafts by locally applied suramin. Circulation. 1999. 100:861–868.
26. Omura T, Yoshiyama M, Izumi Y, et al. Involvement of c-Jun NH2 terminal kinase and p38MAPK in rapamycin-mediated inhibition of neointimal formation in rat carotid arteries. J Cardiovasc Pharmacol. 2005. 46:519–525.
27. Scott NA, Cipolla GD, Ross CE, et al. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996. 93:2178–2187.
28. Currier JW, Faxon DP. Restenosis after percutaneous transluminal coronary angioplasty: have we been aiming at the wrong target? J Am Coll Cardiol. 1995. 25:516–520.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download