Introduction
Previous reports have shown that thoracic aortic atherosclerosis correlates with systemic embolism and vascular disease and is a marker of coronary artery disease.1-3) Transesophageal echocardiography (TEE) provides high resolution imaging of the thoracic aorta and is a reliable tool for evaluating the degree of thoracic aortic atherosclerosis.4)5)
Blankenhorn et al.6) reported that atherosclerosis consists of two components-atherosis and sclerosis-and that future studies should be directed toward evaluating both. TEE is capable of evaluating atherosis and sclerosis simultaneously.
Therefore, it is a very useful tool for evaluating thoracic aortic atherosclerosis.4) Moreover, TEE can provide direct visualization through Doppler technique,7) coronary arterial flow reserve,8)9) and coronary sinus flow reserve.10-12)
This review focuses on TEE evaluation of thoracic aortic atherosclerosis and coronary atherosclerosis and details some of the data we have collected.
Evaluation of Thoracic Aortic Atherosclerosis
TEE has made relatively noninvasive, clear visualization of the aortic arch and descending aorta possible. Therefore, this procedure can be used to select patients at high risk for stroke. Some prospective studies have demonstrated TEE finding of large atheromas (4-5 mm) with mobile attachments are strongly correlated with future embolic disease.13)14) In addition to looking for embolic sources like thoracic atheromas, it is clinically important to evaluate atherosis and sclerosis of the thoracic aorta using TEE. In general, atherosis is evaluated based on intima-media complex thickness (IMT), and sclerosis is evaluated based on arterial stiffness. Atherosis (increased IMT) and sclerosis (increased arterial stiffness) are correlated with generalized atherosclerosis,15) and both are strong predictors of ischemic stroke.16) These parameters are usually evaluated in the carotid artery,15)16) but they can also be evaluated in the aorta.
Measurement of thoracic aortic atherosis
Of the various noninvasive imaging methods available, carotid IMT measurement obtained with B-mode ultrasound is the most widely used method and is recommended by the American Heart Association for evaluation of risk.17) This index was proposed by Pignoli et al.,18) and the authors of the current study applied it to the aorta using TEE.4)19) We then performed pathological examination to determine if IMT as determined by TEE can accurately demonstrate pathological IMT in autopsy cases.4) According to the pathological findings, we believe that IMT can be applied to aortic atherosclerosis by means of TEE.
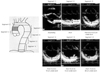
In our previous study, we determined maximum IMTs in 6 segments, according to Pignoli's method (Fig. 1).18) The mean maximum IMT among the 6 segments was used as an index of aortic atherosis. We discovered that dyslipidemia and age were significantly and independently correlated with atherosis, but age was not.4) In addition, Tomochika et al.20) reported that, in patients with familial hypercholesterolemia, cholesterol-lowering therapies could lead to regression of aortic atherosis, using a similar method. This finding can be partially connected to several recent intravascular ultrasound studies showing the effects of cholesterol-lowering therapies on coronary artery plaque regression.21-23) Furthermore, maximum IMT as evaluated by TEE has contributed to the search for new lipid serum markers of aortic atherosis. The authors found that lipoprotein (a) is a significant independent risk factor for aortic atherosclerosis.19) There is a close homology between apolipoprotein (a) and plasminogen,24) so both may play a role in thrombosis and atherosclerosis. One decade after our report detailed above, Peltier et al. supported our data in a study using a similar TEE method in a larger population.25) However, because lipoprotein (a)-lowering medications have not been developed, and because the correlation between coronary atherosclerosis and lipoprotein (a) is controversial,26-28) medical intervention to decrease lipoprotein (a) has not progressed in the meantime. The other serum marker of aortic atherosclerosis that was found using this method was fibrinogen,29) but it was not applied to therapeutic strategies for aortic atherosclerosis.
Measurement of thoracic aortic sclerosis
Sclerosis was clinically measured using three ultrasound methods: stiffness parameter β,30) distensibility,31) and strain parameters.32) The formulae for these parameters are as follows:
For the assessment of aortic sclerosis, the authors principally used stiffness parameter β. The formulae for the other parameters are straightforward, but the stiffness parameter β is supported by several basic science and engineering studies30)33) and has already been applied in many clinical studies.4)20)34) Some reports30)33) have addressed the mechanical behavior of human arterial walls by noting changes in external radii due to distending pressure. These reports have demonstrated that the stiffness parameter β is the slope of the exponential function between the logarithm of the relative arterial pressure and the distension ratio of the artery. This parameter characterizes the full deformation behavior of the vascular wall and is independent of the intramural pressure within the physiological range. Therefore, it indicates aortic sclerosis itself. In the interest of measuring the stiffness parameter β, instantaneous dimensional variables of the aorta (descending aorta) were evaluated. The dimensional changes of the sites with intimal echo were simultaneously recorded during cardiac cycles using M-mode TEE, while a transverse 2D echogram of the descending aorta was recorded at a depth of 15 cm under the arch. Blood pressure was measured at the same time. The minimum aortic dimension during the preejection period (Dd), maximum aortic dimension during the ejection period (Ds), and systolic distension (Ds-Dd) measurements were determined in millimeters. The stiffness parameter β was calculated using the above-mentioned formula (Fig. 2).
Many reports on aortic atherosclerosis have depicted only the grade of aortic atheroma. However, measurement of both the aortic atherosis and sclerosis parameters would improve the characterization of atherosclerosis in the aorta and serve as a more sensitive marker of atherosclerosis progression and regression in clinical trials, compared to atheroma (IMT) measurement alone. Arterial stiffness is related to structural and anatomic changes such as increased collagen to elastin ratio and qualitative deficiency of wall elements. Excluding age, aortic atherosis is significantly correlated with dyslipidemia and diabetes mellitus,4)34) while aortic sclerosis is correlated with hypertension.4) Atherosis and sclerosis seem to represent different atherosclerotic vessel wall properties.4)16)20) Nevertheless, the correlation between the two is weak, but significant.4) Atherosis and sclerosis are two independent processes.15)16) Therefore, TEE is one of the best methods for evaluating aortic atherosclerosis because it can demonstrate aortic atherosis and sclerosis simultaneously.
Possible new index of aortic atherosclerosis
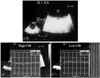
The measurement of integrated backscatter (IBS) is based on the use of unprocessed radiofrequency signals to derive quantitative ultrasonic indices with which to differentiate normal and pathological myocardial structures.35) Quantitative ultrasonic tissue characterization of the biophysical composition of plaque and vessel wall has been performed with high-frequency, high-resolution acoustic microscopy in vitro,36) but such tools are available only in an experimental setting. Acoustic densitometry is a clinically applicable ultrasonic backscatter imaging technology that provides an integrated on-line capability to measure, display, and analyze the average acoustic image intensity within a region of interest (ROI). Thus, with acoustic densitometry, IBS offers a clinically promising method for assessing myocardial contractile performance independent of wall motion37)38) or quantitative evaluation of the vessel wall plaque composition: fibrosis, calcification, or lipid deposition.39)40) The latter approach can provide for a new evaluation of thoracic aortic atherosclerosis. Wickline et al.36) have shown that IBS can be applied to aortic atherosclerosis in vivo. However, it is not known if these tools can be applied in humans. The authors, therefore, tried to use this imaging technique to evaluate thoracic aortic atherosclerosis by means of TEE.41) We placed circular ROIs in the intima-media complex of the aortic wall and vessel lumen and measured the average power of the IBS signal contained with the ROIs. We expressed the relative IBS values of the intima-media complex of the aortic wall as the difference from the IBS values obtained from a reference ROI placed in the vessel lumen {CIB: calibrated IBS value (dB)} (Fig. 3). As a baseline measurement, the authors compared ultrasonic tissue characterization data for excised human aorta specimens with histological findings: fibrofatty or fibrosis tissue specimen. In a trunk filled with a saline solution, we evaluated 24 sections of aorta in which TEE examinations were delimited by a metallic pin: this marker remained fixed in the specimens so the same segment that was imaged by TEE could be evaluated pathologically (Fig. 4). Interestingly, the CIB of the fibrofatty change group (n=14) was significantly lower than that of the fibrous change group (n=10) (23.2±3.2 vs. 31.0±5.7, p<0.001). There were no significant differences in the IMT values for the two groups. Furthermore, we noted a significant, though weak, relation between CIB and stiffness parameter β (y=0.065x+1.47, r=0.50, p=0.003). The correlation between CIB and IMT was not significant. The above-mentioned phenomenon was partially attributable to the fact that IMT indicated a morphological change of aortic atherosclerosis, and stiffness parameter β was shown to be a functional change (including distribution of elastin and collagen of the wall elements). We believe CIB can serve as a new index of aortic atherosclerosis, obtained by TEE. However, IBS has not been established as a standard reference. The authors have used the IBS signal from the vessel lumen as a reference object, while other investigators have used left ventricular cavity,41) myocardium,42) pericardium,43) and adventitia39) as references. The established standard reference tissue for quantitative ultrasonic tissue characterization with IBS analysis is indispensable when this index is used as a 'noninvasive ultrasonic biopsy' in the clinical setting.
Tissue Doppler imaging and strain imaging can be used to evaluate thoracic aortic stiffness. Vitarelli et al.44)45) assessed the aortic elastic properties using tissue Doppler imaging and strain rate imaging in patients with Marfan syndrome and those with a history of coarctoplasty. They found that both groups of patients had abnormal thoracic aortic elastic properties. These new technologies combined with TEE may spread in the field of thoracic aortic atherosclerosis research.
Evaluation of Coronary Atherosclerosis
In 1988, Zwicky et al.46) became the first to report that the coronary arteries could be visualized using color-coded TEE. Since that time, improvements in TEE technology-including multiplane TEE,47) digital imaging,48) and contrast agents49)50) -have made coronary artery visualization clearer, especially with respect to stenosis (atherosclerosis).9)
Visualization of coronary artery stenosis or occlusion
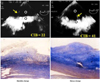
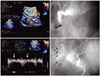
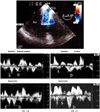
The TEE approach, in which the proximity of the transducer to the coronary artery serves as an advantage, may be useful in evaluating or detecting coronary artery stenosis of the left main coronary artery (LMCA) and proximal left anterior descending coronary artery (LAD).7) A combination of Doppler color flow mapping improves the detection for LMCA and LAD proximal stenosis on TEE.7) In addition, the development of multiplane TEE,47) contrast agents,49)50) second harmonic imaging techniques, 51) and three-dimensional TEE52) has increased the accuracy and efficacy of coronary artery imaging on TEE. Coronary flow velocity can be measured by pulsed Doppler technique, and TEE can demonstrate the proximal segments of the LAD clearly.9) It has already been shown that transthoracic echocardiography with a relatively low velocity range setting (approximately 25 cm/sec) can visualize distal LAD flow.53) Investigators have reported that LAD distal flow can be detected by TTE in 94% of patients,53) but TTE mainly visualizes distal LAD flow. Some investigators have shown that the left circumflex coronary artery (LCX)54) and right coronary artery (RCA)55) can be evaluated by transthoracic echocardiography with contrast agents. However, the detection of coronary flow is not satisfactory in the clinical setting. TEE is obviously superior to TTE in the evaluation of the coronary arteries, mainly due to image quality. However, TEE is semi-invasive. If a small intravenous injection of sedative agents is used, almost all patients can accept this procedure without significant discomfort. Accordingly, in the authors' laboratory, 43 patients underwent TEE with a 5-MHz multiplane transesophageal probe after sedation with a small intravenous injection of propofol. The LAD, LCX, and RCA were evaluated in each patient before and after injection of Levovist (300 mg/mL in 2 mL). Detection of color flow in the LAD, LCX, and RCA was 100%. Coronary flow detection by pulsed Doppler technique was 100%, 77%, and 82% for the LAD, LCX, and RCA, respectively. Therefore, contrast-enhanced multiplane TEE can be used to evaluate three major coronary arteries in the clinical setting. Moreover, detection of total coronary artery occlusion can be performed using echocardiography. It has been reported that patients with total coronary occlusion have a high incidence of cardiac events56) and may benefit from mechanical revascularization.57) Invasive coronary angiography is a definitive diagnostic tool for detecting total coronary occlusion. However, it has been reported that total LAD occlusion can be detected on TTE.58) These reports have shown that the sensitivity and specificity of TTE in identifying LAD occlusion are almost 100%.58)59) However, it is very difficult to apply this TTE technique to the LCX or RCA. It has been reported that only the distal RCA can be evaluated using this technique,60) but due to image quality, anatomy, and the small echo-windows seen in the intercostal area, this technique has many limitations in the evaluation of the LCX and RCA. Thus, the authors evaluated whether this technique could be applied to three major coronary arteries (LAD, LCX, and RCA) using contrast-enhanced multiplane TEE. Seven patients with total proximal coronary occlusion (2 with LAD occlusion, 3 with LCX occlusion, and 2 with RCA occlusion) were evaluated using multiplane TEE. All patients were evaluated by contrast-enhanced TEE. In addition to retrograde flow detection, contrast-enhanced TEE can directly visualize the proximal occlusion site in the coronary artery. TEE can provide clear visualization of almost all of the proximal coronary vessel wall (LAD, LCX, and RCA), so abrupt disappearance of color Doppler flow in a coronary artery suggests total occlusion of the proximal coronary artery. A representative case of total RCA occlusion is shown in Fig. 5. TEE revealed almost no color Doppler flow just proximal to the segment of the RCA considered to be the site of occlusion. Retrograde flow in the RCA suggested functional occlusion of this area. Another representative case with total LCX occlusion is shown in Fig. 6. The abrupt disappearance of color Doppler flow in the LCX was considered to represent the total occlusion; this corresponded with the coronary angiogram results. Contrast-enhanced multiplane TEE can detect proximal LAD, LCX, and RCA functional occlusion in the clinical setting.
Coronary flow reserve
Several reports have shown that CFR measured by TEE is useful in the assessment of significant LAD stenosis.8)61) CFR is usually expressed as the ratio of coronary flow under maximal vasodilation to coronary flow under resting conditions. In the measurement of CFR, vasodilating agents, dipyridamole,61) adenosine,8) and adenosine triphosphate62) have been used. Coronary flow velocities are measured before and immediately after peak vasodilation activity is achieved, and the CFR is expressed as the ratio of the peak diastolic flow velocity during maximal vasodilation to the basal diastolic velocity. Reported TEE cut-off values to predict ischemia span 2.18) to 2.3.61) CFR is now relatively easily measured by TTE, which more clinicians are now using. According to several studies concerning significant LAD stenosis on TTE, the suitable cut-off value of CFR is considered to be 2.0.53)55)63) TTE is superior to TEE in the evaluation of LAD ischemia with CFR, because the former is a completely noninvasive method. However, it is difficult to measure the CFR of the LCX or RCA using TTE, and very few reports have been published concerning the RCA.64)65)
CFR is influenced not only by hemodynamically significant stenosis of the coronary arteries (stenosis >70%), but also by other physiological variables, including myocardial hypertrophy,66) microvascular disease,67) aortic stenosis,68) and left bundle branch block.69) Youn et al.70) reported that CFR using TTE is useful for the detection of microvasculature-induced ischemia; other investigators have reported on the clinical impact of CFR by TTE in the setting of cardiomyopathy, a kind of microvascular disease.71-73) However, CFR using TTE has mainly been focused on the LAD. Simultaneous measurement of the CFR of the LAD, LCX, and RCA requires considerable time, and this technique is not suitable in clinical settings. On the other hand, the authors12) of the current study and some other investigators10)11) have described noninvasive assessment of CFR using transesophageal Doppler evaluation of coronary sinus flow. Calculations based on coronary sinus flow may better represent the CFR of the entire coronary artery system, compared to CFRs of the LAD, LCX, or RCA. Therefore, the authors believe the CFR of coronary sinus flow is superior in evaluating microvascular disease. In order to obtain coronary sinus flow recordings, the TEE probe was advanced to the gastric level, and with the transducer at a dorsal angulation, the probe was cautiously withdrawn until a modified four-chamber view was achieved with visualization of the coronary sinus near its ostium into the right atrium. The transducer position was then optimized to obtain an angle of <30° between the Doppler beam and the long axis of the coronary sinus and to achieve continuous visualization of the vessel throughout the cardiac cycle. Flow recordings were performed after placement of a sample volume into the coronary sinus no more than 1.5 cm from its ostium (Fig. 7).
Compared to TTE, TEE makes it much easier to detect and measure coronary sinus flow. The authors investigated whether the CFR of coronary sinus flow, which can be measured by TEE (especially contrast-enhanced TEE), was useful in evaluating diabetic microvascular dysfunction. We found that, using 1.7 of CFR as a cut-off value, diabetic microvascular dysfunction could be detected with 82% sensitivity and 83% specificity.12) Zehetgruber et al.10) has also reported on the clinical usefulness of the CFR of coronary sinus flow in other microvascular diseases. Therefore, in order to evaluate microvascular disease, the CFR of coronary sinus flow using TEE procedures may be a clinically useful index.
Conclusion
The major clinical role for TEE is the detection of cardiac emboli in patients with stroke and atrial fibrillation, or detailed evaluation of valvular disease. However, TEE has many advantages in the evaluation of thoracic aortic atherosclerosis and coronary atherosclerosis. Clinical TEE measurements should include detailed evaluation of atherosclerosis, with special attention directed to the thoracic aorta and coronary arteries.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download