Abstract
Background and Objectives
Cardiac ankyrin repeat protein (CARP) is an embryonic nuclear protein, and its expression is increased under conditions of pressure or volume overload and also in the failing heart. Adriamycin is a cardiotoxic chemotherapeutic agent, and it suppresses the expression of CARP. We compared the expressions of CARP in the myocardium of normotensive rats and spontaneously hypertensive rats (SHRs) that suffered with adriamycin-induced cardiomyopathy.
Materials and Methods
36 Wistar-Kyoto rats (WKYs) and 36 SHRs were divided into the adriamycin-administered and saline-administered groups. Adriamycin (2.5 mg/kg) and saline were injected intraperitoneally twice a week for 3 weeks. All the animals were sacrificed 3 weeks after the last injection. Immunohistochemical staining was performed on the left ventricles with using synthesized polyclonal CARP antibody. The positively stained areas were measured by using an image analysis program, and the CARP volume fractions (CaVF) were calculated.
Results
CARP was diffusely expressed in the cytoplasm of the myocytes in all the groups. The number of CARP expressing cells was increased in the SHRs. The CaVFs was 5.96±5.11% in the WKYs and it was 9.04±6.26% in the SHRs (p=0.014). The CaVF was 2.26±4.74% in the adriamycin-administered WKYs and it was 1.24±4.32% in the adriamycin-administered SHRs (p=0.32). The adriamycin-administered WKYs and SHRs showed significantly decreased CARP expressions, as compared to the saline-administered groups (p<0.001 and p<0.001, respectively).
Hypertension is a very common disease in the Korean population. Thirty-nine point eight percent of the males and 30.6% of the females older than 30 years in Korea are estimated to be pre-hypertensive. Thirty-four point four percent of the Korean males and 26.5% of the Korean females can be classified as patients if hypertensive patients are defined as those individuals whose systolic blood pressure is higher than 140 mmHg, their diastolic blood pressure is higher than 90 mmHg or they are taking antihypertensive agents.1) This high incidence of hypertension increases the possibility of hypertensive patients having other diseases, and in fact hypertensive patients who are receiving chemotherapy for the treatment of cancer are relatively common. Adriamycin (AD) is a cardiotoxic chemotherapeutic agent that induces cardiomyopathy,2) and hypertension has been shown to be one of the risk factors for cardiomyopathy.3) It has been reported that there is increased perivascular and interstitial fibrosis within the myocardium of an animal model of adriamycin-induced cardiomyopathy with using spontaneously hypertensive rats.4) Yet the role of hypertension on the mechanism of developing adriamycininduced cardiomyopathy has not yet been elucidated.
Cardiac ankyrin repeat protein (CARP) is a nuclear protein that's expressed in the embryonic heart. The CARP expression is increased under conditions of pressure and volume overload and also in a failing heart.5-7) The expression of CARP is noticeably reduced by anthacyclin,8)9) and this suggests that CARP may have a role in the pathogenesis of adriamycin-induced cardiomyopathy.
In this study, we compared the expression patterns of CARP in the myocardium of normotensive rats and spontaneously hypertensive rats. Adriamycin was injected into the animals of each rat group and we compared the expression pattern of CARP before and after the injections.
We considered the characterized 3-dimensional structure of CARP, and we confirmed that the amino acid sequences from 9 to 23 and from 92 to 106 are relatively externally exposed, and so we synthesized these peptides. 10 to 12 weeks old New Zealand white rabbits were prepared, and an immune reaction was induced by subcutaneously injecting the synthesized peptides into the rabbits for a total of 3 times at an interval of 3 weeks at 400 ug per injection. For the primary immunization, 500 µL of Freund's complete adjuvant was mixed with 500 µL of peptide solution, and this was injected subcutaneously at equal amounts to 5-6 sites on the back of the rabbits. From the second immunization, Freund's incomplete adjuvant was used and the mixed solution (the peptides and Freund's incomplete adjuvant) was injected by the same method that was used for the primary immunization. Blood was drawn from the rabbit's ear vein at the time points one week after every peptide injection. An ELISA test was performed with the rabbit's serum to confirm whether the anti-CARP antibody was synthesized by an immune reaction. Immunohistochemical staining was performed on 293 T cells, which were induced to show a CARP expression, to confirm that the prepared anti-CARP antibody does indeed detect the CARP within the 293 T cells. First, we cloned the rat CARP cDNA, and then Polymerase chain reaction (PCR) was performed to amplify the rat CARP DNA. After the sequence of the amplified CARP DNA was confirmed, it was inserted at the Not1 site of the pCR3.1 expression vector. The 293 T cells were transfected with this expression vector and CARP was expressed within the transfected 293 T cells. Immunohistochemical staining was performed with using the anti-CARP antibody obtained after the third immunization (Fig. 1). In addition, these cells were lysed and Western blotting was then performed to confirm that the prepared antibody does specifically detect the CARP (Fig. 2).
Thirty-six male Wistar-Kyoto rats (WKY) weighing 176.8±11.4 g each and 36 male spontaneously hypertensive rats (SHR) weighing 182.8±7.5 g each were divided to 4 groups (all the animals were 6 weeks old). The experiment group was 24 WKYs and 24 SHRs that were treated with adriamycin (WKY-AD, SHR-AD) and 12 WKYs and 12 SHRs that were treated with saline made up the control group (WKY-NS, SHR-NS).
2.5 mg/kg adriamycin (Pharmacia- Upjohn, Peapack, New Jersey, USA) was injected intraperitoneally to the adriamycin administered groups (the WKY-AD and SHR-AD groups), 2 times a week for 3 weeks, and a total of 15 mg/kg was administered. For the saline administration groups (the WKY-NS and SHR-NS groups), the same volume of saline was intraperitoneally injected by the identical method and at the same frequency (Fig. 3).
The blood pressure of the rats was measured, without anesthesia, with using a rat tail blood pressure cuff (KN-210, Natsume Seisakusho Co. Ltd., Tokyo, Japan), prior to performing echocardiography.
Echocardiogrphy was performed on all the animals of all the groups prior to the administration of drugs and then it was performed again 3 weeks after the last administration of drugs. 10 mg/kg Xylazine (BayerKorea, Seoul, Korea) and 100 mg/kg ketamine hydrochloride (Yoohan Yanghang, Seoul, Korea) were injected intramuscularly, and the fractional shortening (FS) was measured using a 15 MHz high frequency probe (Hewlett-Packard 5500, Palo Alto, California, USA).
The animals were sacrificed after the last echocardiography was done. They were anesthetized with ether inhalation, the chest was opened, the heart was exposed, diastolic cardiac arrest was induced by injecting potassium chloride and the heart was then extracted. The extracted heart was dissected, and the left ventricle was divided into 3 tissue pieces by cutting in parallel to the artrioventricular junction. The middle part was fixed in 10% formalin solution and then it was mounted after a dehydration process.
Immunohistochemical staining on the myocardium was performed with using the prepared anti-CARP antibody. The paraffin-embedded tissues were sectioned 4 um in thickness; the sections were placed on a slide and then deparaffined. Enzymatic action with pepsin (Zymed, San Francisco, California, USA) was induced to recover the antigens, and the endogenous peroxidase was blocked by treating the slides with hydrogen peroxide blocking solution (Labvision, Fremont, California, USA).
The slides were reacted with 10% normal goat serum (Zymed, San Francisco, California, USA) for 30 minutes, and then they underwent a primary antibody reaction at 4℃ for 17 hours with using the anti-CARP antibody that was diluted to 1:2,000. A secondary antibody reaction was performed for 30 minutes with HRP goat anti-rabbit IgG (H+L) conjugate (Zymed, San Francisco, California, USA) that was diluted to 1:400. The staining reaction was induced by Diaminobenzidine (Zymed, San Francisco, California, USA). The tissues were stained with Mayer's Hematoxylin (Labvision, Fremont, California, USA). The expression pattern of CARP was examined under a light microscope.
Pictures of the myocardium were taken under a light microscope to quantitate the CARP detected by immunohistochemical staining. The area with stained CARP was measured at 100X magnification with using an Image-pro plus 4.5 (MediaCybernetics, Silver Spring, Maryland, USA) (Fig. 4). By calculating the total area of the cells and the area that was stained for CARP, the CARP volume fraction (CaVF) of each group was then calculated and compared.
The CaVF of each group was compared by paired t-tests and using the Statistical Package for Social Science (SPSS) for windows 11.5 statistical program (SPSS Inc, Chicago, Illinois, USA). All the values are presented as means±standard deviations, and p<0.05 are considered to be a statistically significant.
Forty-eight out of the 72 total animals survived (survival rate: 66.7%). Eleven out of the 12 WKY-NSs and 10 out of the 12 SHR-NSs survived (survival rate: 91.7% and 83.3%, respectively). Seventeen out of the 24 WKY-ADs and 11 out of the 24 SHR-ADs survived (survival rate: 70.8% and 45.8%, respectively). Similar to a previous study,4) there was no difference of blood pressure for each group before and after the administration of adriamycin. The blood pressure of the hypertensive groups (the SHR-NS and SHR-AD groups) was significantly higher than that of the normotensive groups (the WKY-NS and WKY-AD groups).
The left ventricles of the adriamycin administered groups (the WKY-AD and SHR-AD groups) were enlarged and their fractional shortening was significantly decreased, as compare to the saline administered group. The comparison between the WKY-AD and SHR-AD groups showed no significant difference. In a previous study,4) in the WKY-NS group, the fractional shortening before and after the administration of saline was 51.9 ±3.8% and 52.5±7.4%, respectively, and in the WKY-AD group the fractional shortening before and after the administration of adriamycin was 49.70±7.0% and 35.3±6.6%, respectively. In the SHR-NS group, the fractional shortening before and after the administration of saline was 51.0±4.0% and 58.1±10.0%, respectively, and in the SHR-AD group the fractional shortening before and after the administration of adriamycin was 51.0±4.0% and 36.4±10.0%, respectively. We obtained quite similar results in our study.
An increased number of cells expressing CARP was observed in the hypertensive groups compared with that in the normotensive groups. The expression of CARP was markedly decreased after the administration of adriamycin in both the WKY-AD and SHR-AD groups (Fig. 5). As a difference from a previous report showing that CARP was a nuclear protein, CARP was diffusely expressed in the cytoplasm of the cardiomyocytes in all the groups, and this expression pattern showed no differences between the groups (Fig. 6).
The CaVF of the WKY-NS group was 5.96±5.11%, and the CaVF of the SHR-NS group was 9.04±6.26%. The CaVF of the WKY-AD group was 2.26±4.74%, and the CaVF of the WKY-AD group was shown to be 1.24±4.32% (Fig. 7). Compared with the groups that were administered saline (the WKY-NS group vs. the SHR-NS group), the expression of CARP was significantly increased in the hypertensive rats (p=0.014), and on comparison with the adriamycin administered groups (the WKY-AD group vs. the SHR-AD group), no significant difference was detected (p=0.32). We observed that the expression of CARP in the adrimycin administered groups was markedly decreased in comparison with that of the saline administered group of hypertensive rats (the SHR-NS group vs. the SHR-AD group) and the normal blood pressure rats (the WKY-NS group vs. the WKY-AD group) (p<0.001 and p<0.001, respectively).
CARP is a nuclear protein that's expressed in the heart during the developmental period.5)8) Adriamycin suppresses its expression,8)9) and thus, CARP was named cardiac adriamycin responsive protein by some investigators.8) After determining the DNA sequence,10) and ankyrin was found to be repeated 4 times, it has since been referred to as cardiac ankyrin repeat protein or CARP. The expression of CARP is mostly heart specific,8) but it has been reported that CARP was expressed during the regeneration process of damaged skeletal muscles11)12) and in the arterial smooth muscle cells of atherosclerosis lesions, and in the vascular endothelial cells and smooth muscle cells during the regeneration process of arteriogenesis.13)14) The expression of CARP is increased in the myocardium of pressure overloaded animals such as spontaneous hypertensive rats, and also in volume overloaded animals such as Dahl salt-sensitive rats,6) and its expression is also increased in human heart failure.7) The function of CARP is not yet clearly known, but it has been suggested that the function of CARP may be to down-regulate the gene expression in cardiac remodeling and ventricular hypertrophy.6) CARP down-regulates the expression of α-actin, troponin C, atrial natriuretic factor and other genes,8) and it interacts with YB-1, which is a transcription factor of heart specific ventricular myosin light chain 2.5) The expression of CARP is regulated by the Nkx2.5 gene, and this gene plays an important role in the development of the heart during the embryonic period. Activin, TGF-β1 and other stimulators increase the CARP expression, and increased CARP suppresses DNA synthesis and cell proliferation.13)14)
Hypertension is a disease with a high incidence worldwide, and it develops in 1/3 of Korean males and in 1/4 of Korean females, and more than half of the elderly Korean population is hypertensive.1) The effect of hypertension on the heart appears as remodeling of the cardiac muscles in the form of myocardial fibrosis and ventricular hypertrophy in response to pressure overloading.15) The myocardial fibrosis that develops during the remodeling process induces not only the morphological alteration and the thickening of the myocardium, but also the dysfunction of the heart such as gradual diastolic dysfunction, systolic dysfunction and conduction disturbances,16) and this myocardial fibrosis accelerates the ischemia of the myocardium due to hemodynamic dysfunction.17) In the studies performed on spontaneous hypertensive rats, it's been recently observed that there is a greater increase of myocardial fibrosis in hypertensive rats than in normotensive rats. Upon the administration of adriamycin, the fibrosis progressed more than that was noted in the untreated group for both the normotensive and hypertensive rats.4) In the above study, fibrosis was most severely induced when adriamycin was administered to the hypertensive group, and this shows that adriamycin accelerates fibrosis.4) This result shows the myocardial fibrosis is another mechanism of adriamycin-induced cardiomyopathy, in addition to the cell damage caused by the generation of free oxygen radicals,18) apoptosis19-21) and other mechanisms. Therefore, if adriamycin and hypertension affect the heart simultaneously, then the myocardial fibrosis is more aggravated than that caused by the action of adriamycin or hypertension alone, and during this process, it is possible that CARP may act as a mediator of these two stimulations.
In this study, we examined the expression pattern of CARP in the myocardium of normotensive rats and hypertensive rats, before and after the administration of adriamycin, to determine the effect of hypertension on the expression of CARP and the effect of adriamycin on the expression of CARP. In previous studies, CARP was quantitated by measuring its mRNA,6)8) and a CARP expression was observed within the tissues of the embryonic heart. Nevertheless, in our study, the 6 weeks old rats were raised for another 6 weeks; immunohistochemical staining was performed on their myocardium, and the expression of CARP was quantitated. Different from those previous studies reporting that CARP is a nuclear protein, CARP was diffusely expressed in the cytoplasm of all the groups. The number of cells expressing CARP was increased in the hypertensive rats, and after the administration of adriamycin, the expression of CARP was decreased in both the normotensive rats and the hypertensive rats. Considering the result of a previous study that the fibrosis was most severe in hypertensive rats that were administered adriamycin,4) it could be anticipated that CARP would also be less expressed in the adriamycinadministered hypertensive group, and in fact, the CaVF of the adriamycin-administered hypertensive group was shown to be less (1.24±4.32%). Although the CaVF of the adriamycin administered normotensive rats and the hypertensive rats showed no significant difference (2.26±4.74% vs. 1.24±4.32%, respectively, p=0.32), the myocardial fibrosis was most severe in the adriamycin administered hypertensive rats, and this means that hypertension is the main factor that causes fibrosis in this condition. It is possible that the fibrosis caused by hypertension progressed without impediment after the expression of CARP was suppressed by adriamycin administration. This appears to be in agreement with the result of a study that anticipated that CARP may have a protective effect on the myocardium by suppressing the expressions of genes during the remodeling process of the myocardium.6)
This study has a few limitations. First, the reason why CARP was expressed in the cytoplasm and not in the nucleus could not be precisely explained. Cultured cells and embryonic heart tissues were used in the previous studies,5)6)8) and so the reason for the different location of the CARP expression may be that these cells and tissues were different from those of an adult rat. One study5) reported that depending on the culture conditions, CARP was not detected on immunohistochemical staining even if it was extracted by western blotting analysis. Thus, the possibility that undetermined experiment factors affected our results can not be excluded. Furthermore, although we could suggest that the increased expression of CARP is closely related to the suppression of the myocardial fibrosis, we could not determine the precise function of CARP in the pathogenesis of adriamycin-induced cardiomyopathy.
This is the first study that has examined the simultaneous effect of hypertension and adriamycin administration on the expression of CARP in the heart with using an adult animal model. In addition, via preparing anti-CARP antibody and examining the intracellular CARP expression by performing immunohistochemical staining, we obtained valuable experience that is needed to perform further studies in the future. Based on the results of our study, we suggest that suppression of the CARP expression in the adriamycin induced cardiomyopathy model of hypertensive rats plays a certain role in accelerating the myocardial fibrosis. Therefore, the cardiac function of hypertensive patients should be closely observed, and especially when they are being treated with adriamycin.
Figures and Tables
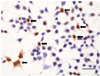 | Fig. 1Immunohistochemical staining of the cultured 293 T cells. The scale bar indicates 25 um. The brown colored cells (black arrow) indicate that the CARP antibody effectively detects the CARP expressing cells. CARP: cardiac ankyrin repeat protein. |
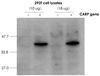 | Fig. 2Western blot analysis of the CARP expression in the 293 T cells. The CARP antibody was shown to specifically recognize CARP. CARP: cardiac ankyrin repeat protein. |
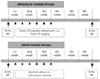 | Fig. 3Schedule of the adriamycin injections and normal saline injections and the echocardiography. Echo: echocardiography, BP: blood pressure, i.p.: intraperitoneally. |
 | Fig. 4Measurement of the CARP expression (WKY-NS). A: the red colored regions represent the CARP positive areas according to the image analysis program (Image Pro-plus 4.5). The scale bar indicates 100 um. B: magnified image of a black rectangle in A. The scale bar indicates 25 um. CARP: cardiac ankyrin repeat protein, WKY-NS: normal saline treated Wistar-Kyoto rat. |
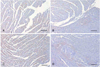 | Fig. 5Immunohistochemical staining of the myocardium. The scale bars indicate 100 um. The brown colored cells are the CARP expressing cells that were detected by the CARP antibody. A: normal saline-treated WKY myocardium. B: adriamycin treated WKY myocardium. C: normal saline treated SHR myocardium. D: adriamycin treated SHR myocardium. CARP: cardiac ankyrin repeat protein, WKY: Wistar-Kyoto rat, SHR: spontaneously hypertensive rat. |
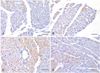 | Fig. 6Immunohistochemical staining of the myocardium. The scale bars indicate 25 um. The brown colored cells are the CARP expressing cells that were detected by the CARP antibody. A: normal saline treated WKY myocardium. B: adriamycin treated WKY myocardium. C: normal saline treated SHR myocardium. D: adriamycin treated SHR myocardium. CARP: cardiac ankyrin repeat protein, WKY: Wistar-Kyoto rat, SHR: spontaneously hypertensive rat. |
Acknowledgments
This study is supported by a grant of The Korean Society of Circulation in 2005 and a grant of the Seoul R & D Program, Republic of Korea (# 10526).
References
1. The Korean Society of Hypertension. Prevalence of hypertension. 2004 Korean Hypertension Treatment Guidelines 2004. 8–15.
2. Takimoto CH. DeVita VT, Hellman S, Rosenberg SA, editors. Topoisomerase interactive agents. Cancer: Principles and Practice of Oncology. 2005. Philadelphia: Lippincott Williams & Wilkins;387.
3. Speyer JL, Ewer MS, Freedberg RS. Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG, editors. Cardiac effects of cancer therapy. Clinical Oncology. 2004. Philadelphia: Elsevier Churchill Livingstone;1252.
4. Uhm JS, Youn HJ, Choi YS, et al. Comparison of adriamycin-induced cardiomyopathy in normotensive rats and spontaneously hypertensive rats. J Korean Soc Hypertens. 2006. 12:23–30.
5. Zou Y, Evans S, Chen J, Kuo HC, Harvey RP, Chien KR. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development. 1997. 124:793–804.
6. Aihara Y, Kurabayashi M, Saito Y, et al. Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promotor. Hypertension. 2000. 36:48–53.
7. Zolk O, Frohme M, Maurer A, et al. Cardiac ankyrin repeat protein, a negative regulator of cardiac gene expression, is augmented in human heart failure. Biochem Biophys Res Commun. 2002. 293:1377–1382.
8. Jeyaseelan R, Poizat C, Baker RK, et al. A novel cardiac-restricted target for doxorubicin: CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem. 1997. 272:22800–22808.
9. Aihara Y, Kurabayashi M, Tanaka T, et al. Doxorubicin represses CARP gene transcription through the generation of oxidative stress in neonatal rat cardiac myocytes: possible role of serine/theronine kinase-dependent pathways. J Mol Cell Cardiol. 2000. 32:1401–1414.
10. Aihara Y, Kurabayashi M, Arai M, Kedes L, Nagai R. Molecular cloning of rabit CARP cDNA and its regulated expression in adriamycin-cardiomyopathy. Biochim Biophys Acta. 1999. 1447:318–324.
11. Baumeister A, Arber S, Caroni P. Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube endcompartments during muscle morphogenesis. J Cell Biol. 1997. 139:1231–1242.
12. Nakada C, Oka A, Nonaka I, et al. Cardiac ankyrin repeat protein is preferentially induced in atrophic myofibers of congenital myopathy and spinal muscular atrophy. Pathol Int. 2003. 53:653–658.
13. de Waard V, van Achterberg TA, Beauchamp NJ, Pannekoek H, de Vries CJ. Cardiac ankyrin repeat protein (CARP) expression in human and murine atherosclerotic lesions: activin induces CARP in smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003. 23:64–68.
14. Boengler K, Pipp F, Fernandez B, Ziegelhoeffer T, Schaper W, Deindl E. Arteriogenesis is associated with an induction of the cardiac ankyrin repeat protein (carp). Cardiovasc Res. 2003. 59:573–581.
15. Weber KT, Jalil JE, Janicki JS, Pick R. Myocardial collagen remodeling in pressure overload hypertrophy: a case for interstitial heart disease. Am J Hypertens. 1989. 2:931–940.
16. Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998. 16:1031–1041.
17. Schwartzkopff B, Motz W, Frenzel H, Vogt M, Knauer S, Strauer BE. Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation. 1993. 88:993–1003.
18. Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000. 207:77–86.
19. Park CS, Youn HJ, Cho EJ, et al. Cardioprotective effect of IGF-1 in mouse with adriamycin induced cardiomyopathy. Korean Circ J. 2002. 32:1116–1123.
20. Choi YS, Park CS, Cho EJ, et al. The relation between acute adriamycin induced cardiomyopathy and apoptosis in rat: study using 15 MHz high frequency transducer. J Korean Soc Echocardiogr. 2002. 10:35–43.
21. Youn HJ, Kim HS, Jeon MH, et al. Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment. Mol Cell Biochem. 2005. 270:13–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download