Abstract
Background and Objectives
We designed this study to determine the therapeutic potentials of umbilical cord blood (UCB)-mesenchymal stem cells (MSCs), as compared with bone marrow (BM)-MSCs.
Materials and Methods
MSCs were isolated from UCB and BM. For the in vivo study, myocardial infarction was induced by ligation of the left anterior descending coronary artery (LAD) in rats for 30 min, and this was followed by release; the MSCs were then injected into a designated point around the infarcted area. Echocardiographs were performed two weeks after surgery. For the in vitro study, a cDNA microarray and cytokine array were performed to compare the MSCs from UCB and from BM. Cell migration was assessed by a wound scratch assay, and the level of cardiac ankyrin repeat protein (CARP) was determined by reverse transcriptase-polymer chain reaction (RT-PCR) or Western blot analysis.
Results
For the echocardiograph findings, the fractional shortening (FS) was 43.9% in the UCB-MSCs group and it was 38.6% in the BM-MSC group. The ejection fraction (EF) was 79.8% in the UCB-MSC group and it was 72.4% in the BM-MSC group (control FS: 26.2% and the control EF: 56.6%). CARP was one of the highly expressed genes in the UCB-MSCs on the cDNA microarray. The mRNA and the expressed level of CARP protein in the UCB-MSCs were higher than those in the BM-MSCs. The cell migration of the CARP small interfering ribonucleic acid (siRNA) transfected UCB-MSCs was delayed compared to that of the normal UCB-MSCs (p<0.05)
A leading cause of heart failure is myocardial ischemia, and this causes the dysfunction and death of cardiomyocytes. 1) Adult stem cells have been proposed to be a promising source for the repair and regeneration of the heart and for restoring the heart's function.2) Several stem cell types such as skeletal myoblasts, hematopoietic stem cells, endothelial progenitor cells and mesenchymal stem cells have been applied to damaged hearts;3-5) however, the optimal cell type remains controversial. Bone marrow (BM) has been widely utilized as a source of autologous stem cells. However, this requires an invasive aspiration procedure to get stem cells from the BM, and the number of cells and their plasticity decreases according to the donors' age or their health condition.6) Due to theses disadvantages of BM for autologous use, there is a need to identify alternative mesenchymal stem cells (MSCs) sources. Stem cells have recently been identified and isolated from umbilical cord blood (UCB), adipose tissue, amniotic membrane etc.7-9) The use of UCB is especially free from ethical problem. Stem cells from UCB have been derived from cord blood just after birth, and they may represent a more potent type of stem cells than those from BM.
The purpose of the present study was to focus on directly comparing the therapeutic potential of MSCs from BM and UCB.
BM was collected from volunteers and the UCB units were from full term (38-40 weeks' gestation) deliveries; the latter were collected from the unborn placenta with obtaining the mother's informed consent for the use of their material for scientific purposes. MSCs were isolated as described previously with slight modification.7) Briefly, to isolate the mononuclear cells (MNC), each BM or UCB unit was diluted 1 : 1 with phosphate-buffered saline (PBS) and then it was carefully loaded onto Ficoll-Hypaque solution (Amersham, Germany). After density gradient centrifugation at 2,500 rpm for 30 minutes at room temperature, the MNCs were collected from interphase and they were washed twice with PBS. The MNCs were seeded at a density 106 cells/cm2 into 6-well culture plates (Falcon, Becton Dickinson, Heidelberg, Germany) in Dulbecco's modified Eagle's medium (DMEM) (Gibco-Invitrogen Corp., USA) that was supplemented by 10% fetal bovine serum (FBS; selected lots; Gibco-Invitrogen), 100 U/mL penicillin and 100 g/mL streptomycin (Sigma-Aldrich, USA). After overnight incubation at 37℃ in a humidified atmosphere containing 5% CO2, the non-adherent cells were removed and fresh medium was added to the wells. The cultures were maintained, and the remaining nonadherent cells were removed by changing the media every 7 days. The culture wells were continuously screened to obtain the developing colonies of adherent cells. The cells were detached by incubating them for 5 minutes in 0.05% trypsin/EDTA (Gibco-Invitrogen, USA), and then they were harvested and replated at a density of 5,000 cells/cm2; these were then termed as passage 1 cells thereafter. Before transplantation into the rat heart, the MSCs were trypsinized, washed and labeled with 50 µg/mL of 4',6-diamidino-2'-phenylindole (DAPI) (Sigma, USA) for 30 minutes.
To analyze the cell-surface expression of typical marker proteins, the MSCs were labeled with anti-human antibodies against HLA-ABC, HLA-DR, CD14, CD29, CD34, CD44, CD45, CD73, CD90, CD105 and CD106 (Becton Dickinson, CA, USA). Mouse isotype antibodies served as the respective controls. Ten thousand labeled cells were acquired and these were analyzed by using a cytomics FC 500 flow cytometer (Beckman Coulter, Krefeld, Germany).
Osteogenic differentiation was achieved by culturing the cells in complete medium along with 0.1 µM dexamethasone, 10 mM β-glycerol phosphate and 50 µM ascorbate. Chondorogenic differentiation was induced by using medium containing 50 µM ascorbic acid, 0.1 µM dexamethazone, 10 ng/mL TGF-β, 40 µg/mL L-proline and 100 µg/mL sodium pyruvate. Adipogenic differentiation was induced by the addition of 0.5 mM isobutylmethylxanthine and 1 µM dexamethasone.
This study was reviewed and approved by the Chonnam National University Institutional Animal Care and Use Committee.
Male Sprague Dawley rats (200-200 g each, Jung Ang Animals, Korea) were anesthetized with an intramuscular injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). Myocardial ischemia-reperfusion injury was induced by temporary ligation of the proximal left anterior descending coronary artery (LAD) for 30 minutes and this was followed by release. The MSCs were directly injected into the peri-infarct areas of the myocardium just before the reperfusion. The sham group rats received PBS (n=15) and the MSC group rats received UCB-MSCs (n=15) or BM-MSCs (n=15) (1×106 diluted in 100 µL of PBS).
The left ventricular (LV) function was assessed by performing transthoracic echocardiography at 2 weeks after surgery with a 7.5-MHz sector scan probe. The rats were anesthetized and placed in the supine position and their chests were shaved. M-mode echocardiograms that were guided by two-dimensional long-axis images were obtained. The LV percent fractional shortening (FS) was calculated as 100×(LVEDD-LVESD)/LV, and the LV ejection fraction (EF) was calculated as 100×(stroke volume/diastolic volume) where the LVEDD is the LV end diastolic diameter and the LVESD is the LV end systolic diameter.
After echocardiography, the rats were sacrificed and their hearts were immediately removed and frozen in liquid nitrogen for immunofluorescent study. Immunofluorescent staining was carried out on the 10-µm frozen ventricular sections.
The samples were incubated overnight with antibodies against α-sarcomeric actin (Sigma, USA), CD31 (BD Science), von Wilebrand factor (Sigma, USA) and connexin 43 (Sigma, USA). The following secondary antibodies were used: anti-rabbit IgG coupled to Alexa Fluor 488 and anti-mouse IgG coupled to Alexa Fluor 594 (InVitrogen, USA). The images were obtained with using a multitracking laser scanning confocal microscope (200×). The fluorochromes were detected at 488 nm and 543 nm.
To compare the gene expression profile of the UCB-MSCs and BM-MSCs, we performed expression analysis by using an Illumina expression chip 24 k (Macrogen, Korea).
To compare the mRNA expression levels in cell-injected animals and the control rats, their myocardial specimens were pulverized and then homogenized in Trizol according to the manufacturer's instructions. cDNA was synthesized to perform reverse transcriptase-polymerase chain reaction (RT-PCR). The primers we used for cardiac ankyrin repeat protein (CARP) were F-ACCGCTATAA GATGATCCGA and R-AATGAAGCTCTGCTCACCAG, and the primers we used for β-actin were, F-GACTACCTCATGAAGATC and R-GATCCACATCTGCTGGAA.
The cells were extracted to separate them on 8% to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then they were transferred to PVDF membranes. The blots were incubated with antibodies against CARP (Santa Cruz, USA) and β-actin (Sigma, USA), and the signals were detected by a LAS-3000 (FUJI, Japan).
The BM-MSCs, UCB-MSCs and the CARP or its scrambled control small interfering ribonucleic acid (siRNA)-transfected UCB-MSCs were cultured on 12-well plates. The confluent cell monolayers were scratched using sterilized 200-µL pipette tips and they were washed with serum free media and then further incubated. The images of the cells were captured at 0 hour, 6 hours and 24 hours after the wounding.
The MSCs were cultured in DMEM supplemented with 1% FBS for 36 hours, and then the cytokine levels in the culture supernatant were detected using the Human Cytokine Antibody Array V (RayBiotech, USA) per the manufacturer's protocol. Briefly, the supernatants were incubated for 2 hours with membranes that were arrayed with antibodies against 79 cytokines. After washing them twice, the membranes were incubated for 2 hours with biotin-conjugated primary nti-cytokine antibodies and then the membranes were washed twice. The membranes were then incubated with horseradish peroxidase-conjugated streptavidin for 1 hour, washed twice and they were placed in the detection buffer for a few minutes. The signals were detected by a LAS-3000 (FUJI,Japan).
The MSCs were characterized by analysis of their surface markers and by the differentiation study. On the cytomeric analysis, both groups of MSCs were positive for CD29, CD44, CD90 and HLA-ABC, while they were both negative for CD13, CD34 and CD106 (Fig. 1). Osteogenic, chondrogenic and adipogenic differentiation were successfully induced (Fig. 1).
To compare the therapeutic capacity of MSCs for repairing the ischemia/reperfusion damage, we injected MSCs into the IR injured rats. Echocardiography was performed at two weeks after IR injury. As shown in Fig. 2, the EF and FS were significantly increased in the UCB-MSCs group and the BM-MSCs group compared with those values of the IR control group (p<0.01). The EF was 79.8±4.6% in the UCB-MSCs group and the EF was 76.1±5.1% in the BM-MSCs group (the IR control: 56.6±6.8%). The FS was 43.9±5.0% in the UCB-MSCs group and it was 40.6±6.3% in the BM-MSCs group (IR control: 26.2±4.2%). The echocardiographic findings did not show any statistical difference between the UCB-MSCs and BM-MSCs groups. The myocardial IR significantly increased the end-diastolic and end-systolic dimensions and it significantly reduced their respective FS and EF.
DAPI-tagging MSCs were detected in the peri-infarct zone at 2 weeks after injection. Immunofluorescence staining showed positively stained cells for α-sarcomeric actin, CD31, von Wilebrand factor and connexin43 in the UCB-MSCs group. On the other hand, only a few cells or none were stained positively in the BM-MSCs group (Fig.3).
The microarray data was examined and the ranking of the genes is shown in Table 1 and 2, which demonstrates the highly expressed genes. Table 3 shows the genes that were more highly expressed in the UCB-MSCs than in the BM-MSCs. This result provided the putative candidate genes to explore for explaining the UCB-MSCs properties as compared with the BM-MSCs. In Fig. 4, a pie chart represents the distribution of the classifications of complementary deoxysibonucleic acid (cDNAs) that were expressed in the UCB-MSCs (A) and BM-MSCs (B). Among the genes that were highly expressed in the UCB-MSCs, we choose CARP and the CARP mRNA level was determined by RT-PCR in the UCB-MSCs and BM-MSCs.
The wound scratching assay was used for the assay of cell migration and motility. Acceleration of wound closure by the UCB-MSCs was observed, as compared to that by the BM-MSCs. To examine whether CARP, which is highly and exclusively expressed in UCB-MSCs (Fig. 5A), contributes to the UCB-MSCs' motility, the CARP was knocked down by using siRNA transfection to the UCB-MSCs. Knock down of the CARP protein level in the siRNA-transfected UCB-MSCs was confirmed by Western blotting, as compared with the non-treated UCB-MSCs (Fig. 5B). The CARP siRNA-transfected UCB-MSCs showed significantly decreased migration of cells into the wounded area (Fig. 5C).
The expression levels of angiogenin, insulin-like growth factor binding protein (IGFBP)-6 and osteoprotegerin were lower in the UCB-MSCs, whereas the levels of most cytokines were similar and that of IGFBP-1 was higher in the UCB-MSCs, as compared to the BM-MSCs (Fig. 6).
In this study, we compared the effects of MSCs from UCB and BM on cardiac IR injured rats, and the gene expression profiles and released cytokines in the MSCs from UCB and BM.
The cardiac function after MI was reported to be significantly recovered by the administration of MSCs from various sources.1)10) Despite that BM has been widely used for applications of cell therapy, aspiration of BM required invasive procedures and the BM isolated from aged or diseased donors has shown impaired cellular functions.
On the other hand, UCB-derived stem cells have several advantages over other adult stem cells, including the ease of harvesting and storage, and the decreased risk for immune intolerance and transmission of infectious agents.11)
Here we report on a comparative study that was performed both in vitro and in cardiac injured rats (IR). Both groups of MSCs exerted a protective effect in IR rats. The EF and FS was a bit more preserved in the UCB-MSCs group; however, there was no significant difference between the UCB-MSC group and BM-MSC group in our study.
Due to the lack of direct evidence that could explain the mechanism by which the MSCs protected the ischemic heart, we tried to compare the gene expression profiles and the therapeutic potentials of the two groups of MSCs from UCB and BM. cDNA microarray analysis revealed that CARP was one of the most highly expressed genes in the UCB-MSCs, as compared with those in the BM-MSCs. CARP has been suggested to act as a nuclear transcription co-factor that negatively regulates the cardiac gene expression and it might play a key role in the pathophysiology of heart failure. Furthermore, CARP is up-regulated in response to shear stress in vitro.12-14) The CARP expression level increases during human heart failure and also in animal models of cardiac hypertrophy, and it is decreased in cardiomyocytes that are exposed to adriamycin.15) These reports suggested that the CARP expression might be associated with pathological stress in cardiomyocytes.
To explore the possible role of CARP in UCB-MSCs, strong cell motility, which is one of the prominent characters of UCB-MSCs as compared with that of BM-MSCs, was utilized as a reference for comparison between UCB-MSCs and the CARP-knock down UCB-MSCs. On the wound scratch assay, the CARP knock down UCB-MSCs migrated more slowly than did the control UCB-MSCs; however, the CARP knock down UCB-MSCs were still faster than the BM-MSCs (data not shown). From this result, CARP can be suggested to be partly responsible for the migration of UCB-MSCs. Strong migration may contribute to the homing process into an injured organ site; however, we did not confirm the better homing capacity of UCB-MSCs in vivo, and a long term observation study on this should be conducted in the future.
There is currently debate on whether implanted MSCs can differentiate into cardiomyocytes or the endothelial phenotype, and if they can enhance cardiac survival and the vascular density after implantation in a rat myocardial infarction model.16)17) More recently, other researchers have reported that grafted stem cells can secrete several important survival factors such as vascular endothelial growth factor, stromal cell-derived factor, basic fibroblast growth factor, hepatocyte growth factor and insulin-like growth factor, and these could protect or salvage endangered ischemic myocardium.18) This paracrine effect may be as important as the differentiation potential of stem cells for achieving functional improvement. However, a significantly high percentage of the transplanted stem cells was lost within a few days, and their survival rate has been reported to be less than 0.44%.19-21) Hence, safe and effective methods to promote stem cell survival need to be developed to perform successful stem cell therapy.
In conclusion, we report here on the differences between UCB-MSCs and BM-MSCs. In these experiments, the UCB-MSCs showed a stronger motility potential, yet there was no significant difference between the UCB-MSCs and BM-MSCs on the in vivo study.
Figures and Tables
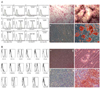 | Fig. 1Mesenchymal stem cells (MSCs) characterization. FACS analysis and a differentiation study were performed on the umbilical cord blood (UCB)-MSCs (A) and the bone marrow (BM)-MSCs (B), and both groups of MSCs expressed the typical MSC markers. Both groups of MSCs (a) were differentiated to osteoblasts (b), chondrocytes (c) and adipocytes (d). FACS: Fluorescence-Activated Cell Sorter. |
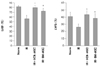 | Fig. 2Echocardiographic parameters. The ejection fraction (EF) and fractional shortening (FS) of the left ventricle in the cardiac ischemia-reperfusion (IR) injured rats that were treated with mesenchymal stem cells. n=15 in each group, *p<0.05 vs. the IR control rats. LVEF: left ventricular ejection fraction, LVFS: left ventricular fractional shortening, UCB: umbilical cord blood, MSC: mesenchymal stem cell, BM: bone marrow. |
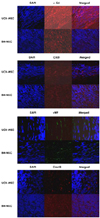 | Fig. 3DAPI-stained mesenchymal stem cells (MSCs) at 14 days. Sections from the engrafted hearts were stained with antibodies against α-sarcomeric actin (α-SA), von Wilebrand factor (vWF), CD31 and connexin43 (Cn×43). Immunofluorescence staining was done to examine if the injected MSCs were differentiated. The sections were stained for α-SA, CD31, vWF and Cn×43. The MSCs were stained with DAPI before injection to detect the region of the myocardium where they homed to. UCB: umbilical cord blood, MSC: mesenchymal stem cell, BM: bone marrow, DAPI: 4'-6-diamidino-2-phenylindole. |
 | Fig. 4cDNA microarray profiles of the umbilical cord blood (UCB)-mesenchymal stem cells (MSCs) and the bone marrow (BM)-MSCs. The pie charts show the percentages of the cDNAs that were expressed in the UCB-MSCs (A) and BM-MSCs (B). cDNA: complementary deoxyribonucleic acid. |
 | Fig. 5Expression of CARP and the cell migration assay of the umbilical cord blood (UCB)-mesenchymal stem cells (MSCs). The higher expression level of CARP in the UCB-MSCs was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot analysis (A). To knock down the endogenous CARP in UCB-MSCs, 100 nM of siRNA was transfected and the reduced CARP protein level was shown on Western blotting (B). Cell migration was assayed by the wound scratch assay. UCB-MSCs were transfected with scrambled siRNA or CARP siRNA to examine the effect of CARP on cell motility (C). CARP: cardiac ankyrin repeat protein, siRNA: small interfering ribonucleic acid. |
 | Fig. 6Cytokine array of the umbilical cord blood (UCB)-mesenchymal stem cells (MSCs) and bone marrow (BM)-MSCs. The different cytokines between the two groups of MSCs are marked on the blots. IGFBP: insulin-like growth factor binding protein. |
Acknowledgments
This work was supported by the Korean Society of Cardiology CA#70NANB7H3061 and the Stem Cell Research Program funded by the Ministry of Science and Technology (MOST), Republic of Korea. (M10641450001-06N4145-0011).
References
1. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.
2. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
3. Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005. 102:11474–11479.
4. Piao H, Youn TJ, Kwon JS, et al. Cellular cardiomyoplsty using bone marrow derived mesenchymal stem cells transplantation in post myocardial infarction heart failure. Korean Circ J. 2004. 34:1113–1121.
5. Lim SY, Jeong MH, Ahn YK, et al. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Korean Circ J. 2005. 35:734–741.
6. Zhang H, Fazel S, Tian H, et al. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005. 289:H2089–H2096.
7. Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004. 22:625–634.
8. Moon MH, Kim SY, Kim YJ, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006. 17:279–290.
9. Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005. 79:528–535.
10. Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006. 12:459–465.
11. Tsai MS, Hwang SM, Chen KD, et al. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells. 2007. 25:2511–2523.
12. Han XJ, Chae JK, Lee MJ, You KR, Lee BH, Kim DG. Involvement of GADD153 and cardiac ankyrin repeat protein in hypoxia-induced apoptosis of H9c2 cells. J Biol Chem. 2005. 280:23122–23129.
13. Nakada C, Oka A, Nonaka I, et al. Cardiac ankyrin repeat protein is preferentially induced in atrophic myofibers of congenital myopathy and spinal muscular atrophy. Pathol Int. 2003. 53:653–658.
14. Shi Y, Reitmaier B, Regenbogen J, et al. CARP, a cardiac ankyrin repeat protein, is up-regulated during wound healing and induces angiogenesis in experimental granulation tissue. Am J Pathol. 2005. 166:303–312.
15. Chen CL, Lin JL, Lai LP, Pan CH, Huang SK, Lin CS. Altered expression of FHL1, CARP, TSC-22 and P311 provide insights into complex transcriptional regulation in pacing-induced atrial fibrillation. Biochim Biophys Acta. 2007. 1772:317–329.
16. Qi CM, Ma GS, Liu NF, et al. Identification and differentiation of magnetically labeled mesenchymal stem cells in vivo in swines with myocardial infarction. Int J Cardiol. 2007. [Epub ahead of print].
17. Numaguchi Y, Sone T, Okumura K, et al. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006. 114:1 suppl. I114–I119.
18. Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005. 80:229–236.
19. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998. 142:1257–1267.
20. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002. 105:93–98.
21. Pagani FD, DerSimonian H, Zawadzka A, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans: histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003. 41:879–888.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download