Abstract
In-stent atheromatous plaque rupture is a very rare event. A 51-year-old man presented with an acute inferior myocardial infarction 9 years after bare-metal stent implantation in the mid-portion of right coronary artery. After thrombolytic therapy, coronary angiography and intravascular ultrasound (IVUS) revealed a ruptured plaque at the mid portion of the stented segment.
Coronary artery disease (CAD) is a leading cause of morbidity and mortality in the United States.1) Advances in pharmacologic and surgical management have improved outcomes in patients with CAD. Restenosis is the most common complication associated with coronary stent implantation. The pathophysiology of restenosis is complex and incompletely understood. Current evidence suggests that restenosis is a maladaptive response of the coronary artery to the trauma induced by angioplasty that causes thrombosis, inflammation, cellular proliferation, and extracellular matrix production that contribute to postprocedural lumen loss within six months.2) Plaque rupture at the site of stent insertion is quite rare; here we report the first Korean case presenting with symptoms of a ST elevation myocardial infarction.
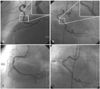
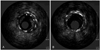
A 51-year-old man presented to the emergency room with chest pain in November 2007. The patient had a history of type 2 diabetes mellitus, hypertension and an acute myocardial infarction in January 1999 when he was admitted to our hospital. At that time, the coronary angiogram showed three vessel disease with 80% stenosis at the mid-portion of right coronary artery (Mid-RCA). A bare-metal stent (Nir 4.0×16 mm) was implanted at the mid-portion of RCA. Follow up angiogram in April 2001, due to recurrent chest pain, showed excellent flow and a patent coronary artery with 20% in stent restenosis at the mid-portion of RCA and 70% tubular stenosis at the mid-portion of left anterior descending coronary artery (Mid-LAD). The patient was medically treated and his condition improved. He did not complain of chest pain afterwards during follow up in the outpatient department. Currently, the patient was admitted to the emergency room with chest pain that started 30 minutes prior to admission. On the basis of the results of a 12-lead electrocardiogram (ECG), the patient was diagnosed with an acute inferior myocardial infarction (AMI). Intravenous tissue type plasminogen activator (tPA) was immediately infused but the chest pain did not subside after one hour. The patient was immediately transferred to the cardiac catheterization laboratory and an angiogram demonstrated a flap like dissection within the stented segment (Fig. 1A and B). No significant stenosis was seen at mid-LAD and proximal compared to the previous angiogram. Angioplasty was performed at the RCA. A 7F JR 4.0 Cordis guiding catheter was positioned at the RCA ostium. An Asahi Rinato guide wire was advanced into the distal RCA. To determine the etiology of the dissected lesion of the mid-RCA, an intravascular ultrasound (IVUS) (Boston Scientific) was performed. The IVUS image demonstrated that the dissected lesion noted on the angiogram was a ruptured atheromatous in stent restenosis site with a thin dissected flap within the stent; the stented vessel size was well preserved (Fig. 2A). These findings suggested the presence of a ruptured plaque from in stent restenosis. The external elastic membrane (EEM) lumen crosssectional area (CSA) was 18.1 mm2, the minimal lumen area was 6.4 mm2, the plaque crosssectional area was 11.6 mm2, and the plaque burden was 64.1%. The minimum lumen diameter was 2.4 mm and the vessel diameter was 4.8 mm. The diameter stenosis was 52.1% at the proximal edge of the stent. A 4.0×24 mm Taxus stent (Boston Scientific) was placed to cover the previous stent and the dissected plaque. Another 4.0×24 mm Taxus stent (Boston scientific) was deployed at the proximal RCA after inflation under a pressure of 9 atm. The post stenting angiogram showed patent flow with no residual stenosis (Fig. 1C and D); the IVUS showed a well expanded stent (Fig. 2B). The patient remained stable and was discharged after 48 hours of observation.
The acute coronary syndrome can be caused by plaque rupture (i.e., by the interaction between blood flow and the intimal surface).2) In cases with an acute myocardial infarction, a fibrin-rich thrombus is present on a disrupted atherosclerotic plaque, whether or not it is clearly visualized by angiography.1) The lesion beneath the thrombus is typically lipid-rich, with a thin fibrous cap. IVUS has the ability to detect plaque rupture that is not detectable by angiography alone.3-5) The lesions that are most prone to postprocedure cardiac events most commonly have a thin fibrous cap and a large lipid core (yellow plaques). Because these types of plaques are also most prone to spontaneous rupture, they presumably have a greater propensity to rupture and become thrombogenic during angioplasty.
Stent placement has been shown to reduce the rate of restenosis compared to balloon angioplasty alone. Patterns of arterial remodeling during the course of plaque development have been shown to play an important role in both the progression of de novo atherosclerosis and during the restenotic process following percutaneous coronary interventions (PCI).6) After stenting is performed, the stent prevents remodeling caused by late vessel-constriction. Restenosis is the result of uniform neointimal tissue proliferation throughout the stent.7) Endovascular stents reduce restenosis by providing the vessel lumen with rigid scaffolding. Although baremetal stents reduce early elastic recoil and lumen loss due to remodeling, they do not prevent the neointimal hyperplasia and may, in fact, amplify the proliferative component of restenosis.
Stent thrombosis is a rare, but very serious and potentially a fatal complication of percutaneous treatment of coronary atherosclerosis.8) The estimated 30-day mortality ranges from 20 to 48%; myocardial infarction in 60-70% of patients.9) Stent thrombosis has been classified as 1) acute, occurring within 48 hours of stent implantation, 2) subacute, occurring anytime between the second day and one month after the procedure and 3) late, corresponding to all cases occurring after the first 30 days following percutaneous interventions. Late stent thrombosis has been observed more frequently following intravascular brachytherapy to treat in stent restenosis compared to intracoronary bare metal stent implantation; this difference is presumed to be related to the vessel size and lesion complexity.
The atherosclerotic plaques associated with thrombosis and total occlusion, located in infarct-related vessels, are generally more complex and irregular than those in vessels not associated with ST-elevation myocardial infration.10) At autopsy, histological studies of these lesions often reveal plaque rupture or erosion. Gavaliatsis was the first to demonstrate coronary plaque rupture in humans by routine coronary angiography in 1996.11) The rupture of an atherosclerotic plaque exposes the thrombogenic contents of the plaque to the flowing blood, triggering platelet activation, adhesion and thrombosis. The case presented here illustrates a plaque rupture, leading to acute myocardial infarction and total in-stent occlusion. After thrombolysis, angiography was performed immediately due to the ongoing chest pain. Even after restoration of thrombolysis in myocardial infarction (TIMI) 3 flow, the symptoms from the ruptured plaque persisted after therapy. IVUS was performed and permitted detailed, high-quality, cross-sectional imaging of the coronary arteries in vivo.7) By means of the serial angiography and IVUS images, we documented the abnormal vessel response to stenting.
In-stent plaque rupture is a rare angiographic phenomenon and secondary prevention of plaque rupture following percutaneous coronary intervention in patients with acute coronary syndrome has not been well studied. Effective intravenous thrombolysis has allowed IVUS to emerge as an informative diagnostic tool for a ruptured coronary plaque. The ability to obtain this information has prompted consideration of interventional efforts for possible prevention of recurrent plaque rupture through procedures such as the use of longer stents to cover diffuse complex lesions, in combination with aggressive risk factor modification, including statin therapy, blood pressure lowering and more prolonged use of antiplatelet agents.12)13) However, the optimal treatment for the prevention of plaque rupture has not been determined to date.
Figures and Tables
References
1. Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007. 100:3K–9K.
2. Forrester JS. Toward understanding the evolution of plaque rupture: correlating vascular pathology with clinical outcomes. J Am Coll Cardiol. 2003. 42:1566–1568.
3. Hong BK, Cho SY, Jang YS, et al. Intravascular ultrasound imaging in patient with acute myocardial infarction. Korean Circ J. 1998. 28:931–938.
4. Okura H, Taguchi H, Kubo T, et al. Atherosclerotic plaque with ultrasonic attenuation affects coronary reflow and infarct size in patients with acute coronary syndrome: an intravascular ultrasound study. Circ J. 2007. 71:648–653.
5. Hur SH, Hassan AH, Rekhi R, et al. Serial intravascular ultrasonic study of outcomes of coronary culprit lesions with plaque rupture following bare metal stent implantation in patients with angina pectoris. Am J Cardiol. 2007. 99:1394–1398.
6. Hong YJ, Jeong MH, Hyun DW, et al. Impact of preinterventional arterial remodeling on in-stent neointimal hyperplasia and in-stent restenosis after coronary stent implantation. Circ J. 2005. 69:414–419.
7. Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996. 94:1247–1254.
8. Feres F, Costa JR Jr, Abizaid A. Very late thrombosis after drug-eluting stents. Catheter Cardiovasc Interv. 2006. 68:83–88.
9. Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent trials. Circulation. 2001. 103:1967–1971.
10. Nallamothu BK, Blaney ME, Morris SM, et al. Acute reperfusion therapy in ST-elevation myocardial infarction from 1994-2003. Am J Med. 2007. 120:693–699.
11. Gavaliatsis IP. From thrombolysis to thrombogenesis in clinical practice: coronary ruptures plaque angiography. Int J Cardiol. 1996. 55:103–105.
12. Scott NA. Restenosis following implantation of bare metal coronary stents: pathophysiology and pathways involved in the vascular response to injury. Adv Drug Deliv Rev. 2006. 58:358–376.
13. Hong MK. Medical treatments to prevent in-stent restenosis. Korean Circ J. 1999. 29:353–356.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download