Abstract
Background and Objectives
Bilirubin has a protective role in suppressing atherosclerosis and coronary artery disease by its potent physiological antioxidant properties. There has been no comparative study on the relation between the bilirubin level and the coronary microvascular function in diabetic patients. This study investigated whether the bilirubin level correlates with the coronary microvascular integrity in diabetes by assessing the coronary flow velocities after successful percutaneous coronary intervention (PCI).
Subjects and Methods
Fifty patients (31 males and 19 females, mean age 60±11) with angina and who received elective PCI were studied. Using an intracoronary Doppler wire, the coronary flow velocity reserve (CFR), the hyperemic microvascular resistance index and the phasic coronary flow velocity patterns were measured after PCI.
Results
The mean value of the fasting blood glucose was 211±88 mg/dL, the man value of glycated hemoglobin A1c (HbA1c) was 8.1±1.6% and the mean serum total bilirubin level was 0.59±0.21 mg/dL. CFR was significantly correlated with the serum bilirubin level (r=0.485, p<0.001), HbA1c (r=-0.432, p=0.003) and the fasting blood glucose (r=-0.361, p=0.011). On multivariate analysis, HbA1c, bilirubin and left ventricular hypertrophy showed independent relationships with coronary microvascular dysfunction (p=0.003, p=0.004, p=0.033, respectively).
Figures and Tables
Fig. 1
The relationship among the microvascular indices, HbA1c and bilirubin. Serum bilirubin is plotted against CFR (A), bAPV (B), bDDT (C) and HbA1c (D). The correlation coefficients are shown. CFR: coronary flow reserve, bAPV: baseline average peak velocity, bDDT: baseline diastolic deceleration time, HbA1c: glycated hemoglobin A1c.

Fig. 2
The receiver operating characteristic (ROC) curve analysis and the adequate cut-off values of bilirubin and HbA1c for a CFR <2. The best cut-off values (BCVs) for a CFR <2 are 0.5 mg/dL (A) and 8.2% (B). HbA1c: hemoglobin A1c, AUC: area under the curve.

Fig. 3
Comparison of the CFR according to the median value of bilirubin and HbA1c. The CFR was higher for a bilirubin level >0.5 mg/dL and a HbA1c=8.1%. CFR: coronary flow reserve, HbA1c: hemoglobin A1c.

Table 3
Univariate and multivariate predictors of CFR among the clinical variables
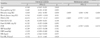
CFR: coronary flow reserve, CI: confidence interval, HbA1c: glycated hemoglobin A1c, CK: creatine kinase, CK-MB: creatine kinase-myocardial band, LVH: left ventricular hypertrophy, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, bpm: beats per minute, MLD: minimal luminal diameter
References
1. Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999. 340:115–126.
2. Rodriguez-Porcel M, Herman J, Chade AR, et al. Lomg-term antioxidant intervention improves myocardial microvascular function in experimental hypertension. Hypertension. 2004. 43:493–498.
3. Zhu XY, Daghini E, Chade AR, et al. Role of oxidative stress in remodelling of myocardial microcirculation in hypertension. Arterioscler Thromb Vasc Biol. 2006. 26:1746–1752.
4. Esterbauer H, Gebicki J, Puhl H, Jungens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992. 13:341–390.
5. Neuzil J, Stokcer R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibitory plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994. 269:16712–16719.
6. Hulea SA, Wasowicz E, Kummerow FA. Inhibition of metal-catalyzed oxidation of low-density lipoprotein by free and albumin-bound bilirubin. Biochem Biophys Acta. 1995. 1259:29–38.
7. Wu TW, Fung KP, Wu J, Yang CC, Weisel RD. Antioxidtion of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol. 1996. 51:859–862.
8. Gibaldi M. Antioxidant vitamins and health. J Clin Pharmacol. 1996. 36:1093–1099.
9. Minetti M, Mallozzi C, Di Stasi AM, Pietraforte D. Bilirubin is an effective antioxidant of peroxynitrite-mediated protein oxidation in human blood plasma. Arch Biochem Biophys. 1998. 352:165–174.
10. Jun JE, Joung HJ, Chun BY, et al. Effects of antioxidant supplementation on lipid peroxidation and antioxidative enzyme activities in patients with coronary heart disease. Korean Circ J. 2001. 31:1215–1224.
11. Koh JH, Ryu KH, Lim SH, et al. Effect of antioxidants on myocardial damage in streptozotocin-induced diabetic rats. Korean Circ J. 2006. 36:261–271.
12. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importances. Science. 1987. 235:1043–1046.
13. Gullu H, Erdogan D, Tok D, et al. High serum bilirubin concentrations preserve coronary flow reserve and coronary microvascular functions. Arterioscler Thromb Vasc Biol. 2005. 25:2289–2294.
14. Nahser PJ Jr, Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995. 91:635–640.
15. Akasaka T, Yoshida K, Hozumi T, et al. Retinopathy identifies marked restriction of coronary flow reserve in patients with diabetes mellitus. J Am Coll Cardiol. 1997. 30:935–941.
16. Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004. 15:259–264.
17. Yoon MH, Tahk SJ, Choi SY, et al. Coronary flow reserve as a predictor of long-term clinical outcome after acute myocardial infarction. Korean Circ J. 2002. 32:756–765.
18. Marks DS, Gudapati S, Prisant LM, et al. Mortality in patients with microvascular disease. J Clin Hypertens. 2004. 6:304–309.
19. Kesavulu MM, Giri R, Kameswara Rao B, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000. 26:387–392.
20. Nitenberg A, Ledoux S, Valensi P, Sachs R, Antony I. Coronary microvascular adaptation to myocardial metabolic demand can be restored by inhibition of iron-catalyzed formation of oxygen free radicals in type 2 diabetic patients. Diabetes. 2002. 51:813–818.
21. Moreno R, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004. 44:2293–2300.
22. Zuanetti G, Latini R, Maggioni AP, Franzosi MG. Influence of diabetes on mortality in acute myocardial infarction: data from the GISSI-2 study. J Am Coll Cardiol. 1993. 22:1788–1794.
23. Sundell J, Laine H, Nuutila P, et al. The effects of insulin and short-term hyperglycemia on myocardial blood flow in young men with uncomplicated Type 1 diabetes. Diabetologia. 2002. 45:775–782.
24. Schnell O. Cardiac sympathetic innervation and blood flow regulation of the diabetic heart. Diabetes Metab Res Rev. 2001. 17:243–245.
25. Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994. 40:18–23.
26. Schwetner HA. Association of smoking and low serum bilirubin antioxidant concentrations. Atherosclerosis. 1998. 136:383–387.
27. Djoussé L, Levy D, Cupples A, Evans JC, D'Agostino RB, Ellison RC. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol. 2001. 87:1196–1200.
28. Clark JA, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischmic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000. 278:H643–H651.
29. Öllinger R, Bilban M, Erat A, et al. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005. 112:1030–1039.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download