Abstract
Background and Objectives
Strain imaging has already been shown to quantify regional myocardial function in both acute ischemic myocardium and infarcted myocardium. We proposed that strain imaging could differentiate deformation of normal and ischemic myocardium that are without regional wall motion abnormality, as assessed by conventional echocardiography. The aim of this study is to determine the diagnostic value of strain imaging for the detection and localization of coronary lesions in patients with chest pain, but they are without apparent wall motion abnormalities.
Subjects and Methods
Strain imaging for advanced wall motion analysis was performed in 179 patients with suspicious stable angina (SA) and in 94 patients with suspicious acute coronary syndrome (ACS) prior to coronary angiography. All the patients had normal conventional wall motion scoring based on the standards of the American Society of Echocardiography. Longitudinal strain was measured in 3 apical views, and assessments of the strain value for individual segments with using an 18-segment division of the left ventricle were performed to determine the average strain value. Marked heterogeneity of strain was considered abnormal, and significant coronary artery disease was considered present if stenosis above 70% was noted on the quantitative angiography.
Results
Eighty (78%) of the 103 patients with SA and 18 (56%) of the 32 patients with ACS and who showed constant systolic strain throughout the left ventricular wall had normal or minimal coronary lesions. Fifty-one (67%) of the 76 patients with SA and 53 (85%) of the 62 patients with ACS and marked heterogeneity of strain had angiographically significant coronary stenosis. The receiver-operating characteristic (ROC) analysis of the peak systolic strain yielded that the ROC-area of peak systolic strain for the left anterior descending artery territory was 0.79 (95% CI 0.72-0.84), this was 0.87 (95% CI 0.79-0.91) for the left circumflex artery territory and 0.89 (95% CI 0.79-0.93) for the right coronary artery territory.
Conclusion
Ultrasound-based strain imaging demonstrates a strong correlation with coronary angiography and it has potential as a noninvasive diagnostic tool for detecting coronary artery stenosis in patients with chest pain, but who are without apparent wall motion abnormalities on conventional echocardiography.
Traditionally, the day-to-day evaluation of regional ischemia is most often based on the visual assessment of wall motion and wall thickening and with the data on wall thinning that's derived from 2D grayscale images. This has well-documented limitations for both the interobserver variability1) and the ability of the human eye to resolve rapid, short-lived motion.2) Another approach to defining the regional myocardial properties could be to evaluate the deformation of a myocardial segment during the cardiac cycle. Two parameters that reflect myocardial deformation properties can be extracted from the cardiac ultrasound data: the regional strain and the strain rate.
The actual sequence of the regional changes in the myocardial function that are induced by acute ischemia has been well defined by experimental sonomicrometric techniques.3-6) Acute ischemia induces a delay in the onset of contraction, a progressive decrease in the rate and degree of thickening, and a progressive delay in the timing of the peak thickening until this event occurs in what is early diastole for the surrounding nonischemic myocardial segments. Finally, systolic thickening is virtually or completely abolished by total occlusion, and only late systolic/early diastolic thinning occurs. Although it has been well documented in the animal laboratory setting, all the components of the above ischemic response have yet to be well documented in the clinical setting by noninvasive imaging techniques. With the introduction of tissue Doppler imaging (TDI), it has also become possible to determine segmental velocities at a sampling rate of more than 140 samples per second by using standard echo views. Prior in vivo animal studies based on TDI have documented a significant reduction in the peak systolic velocities, the velocity gradient7)8) and the peak systolic strain9)10) that occur during acute ischemia. Thus, the quantitation of the segmental systolic parameters derived from high-resolution TDI data might be the optimal solution for functional studies of patients with coronary artery disease. In this investigation, we proposed to evaluate the relative diagnostic value of the strain parameters for detecting acute ischemic changes in the myocardium with normal wall motion scores on conventional echocardiography. Our goals were to determine whether the strain parameters would help detect ischemia at rest and if these parameters could present useful information before performing coronary angiography.
This study's subjects were prospectively enrolled between May 2004 and April 2005. We studied 189 consecutive patients with suspected stable angina (SA) (85 men and 104 women, mean age: 59±12 years) and 110 patients with suspected acute coronary syndrome (ACS) (62 men and 48 women, mean age: 60±9 years) for whom elective coronary angiography was planned. All the patients had global normal conventional wall motion scoring based on the standards of the American Society of Echocardiography. Patients with a prior history or electrocardiogram (ECG) signs of transmural myocardial infarction, dilated cardiomyopathy, myocardial hypertrophy, significant valve disease, atrial or ventricular arrhythmia, pacemaker implantation, bundle brunch blocks, apparent wall motion abnormality or a left ventricular ejection fraction less than 50% were not included in the study. Echocardiography ruled out concomitant hypertrophic cardiomyopathy in all the patients. The coronary angiography was quantitatively analyzed, and significant coronary artery disease was defined if the stenosis was more than 70% of the lumen diameter. The normal coronary group was defined as there was no stenosis or the stenosis was <50% of the lumen diameter. If the patients had stenosis that was more than 50% and under 70%, then they were not included in the study. The 10 patients with SA and 16 patients with ACS were excluded. Institutional review board approval was obtained, and all the subjects provided signed informed consent. The demographic data, including age, gender and the cardiovascular risk factors, were recorded.
Echocardiographic studies were performed with a Vingmed System 7 Dimension (GE Vingmed, Horten, Norway) and a 3.5 MHz transducer. All the patients were scanned in the lateral decubitus position, and the routine 2D grayscale and tissue Doppler images were recorded at 3 apical views prior to coronary angiography. The left ventricular chamber size was obtained in the M-mode, and the ejection fraction was determined using the modified Simpson method. End-diastole and end-systole were defined to occur at the R peak on the electrocardiographic tracing and aortic valve closure was defined to occur on the Doppler profile, respectively. Those cardiac cycles associated with atrial and/or ventricular extrasystolic beats, post-extrasystolic cycles or any other rhythm abnormalities were excluded. Cineloops of 2 to 3 cardiac cycles from the anterior, lateral, posterior, inferior, septal, and anteroseptal walls were acquired separately at end-expiratory apnea and these images were digitally stored at 50 frames/s for subsequent visual analysis. We used the narrowest possible image sector angle to achieve the maximum color Doppler frame rate (range: 70 to 164, and typically 141 frames/s). We performed real-time analysis of the longitudinal peak systolic strain of the individual segments using an 18-segment division of the left ventricle (each wall in each apical view was divided into the basal, middle and apical segments). Two parameters were used for the identification of ischemia. The first was a magnitude parameter, being defined as a marked reduction of the peak systolic strain, or a flattened pattern of strain. The second included a temporal component, which was based on the recognition of post-systolic shortening and this was identified visually by comparing the strain color maps in early diastole with the strain color maps in mid-systole. Marked heterogeneity of strain was considered abnormal. These segments were called the strain-positive segments, and the rest of the segments were called strain negative. A homogenous pattern or constant strain was defined as relatively uniform distribution of the peak systolic strain with no occurrence of post-systolic shortening. We assigned the segments of the left ventricle to each vascular territory as follows: the left anterior descending (LAD) artery territory that included the anteroseptal, anterior and mid-inferior septal segments; the left circumflex (LCx) artery territory that included the lateral and posterior segments, and the right coronary artery (RCA) territory that included the basal inferior septum and inferior segments.
All the data is expressed as means±standard deviations (SDs). The data was analyzed using standard statistical software (Statistical Package for Social Science; SPSS package version 11.0), and comparisons of all measurements were done with a paired Student's t-test for the continuous variables and a chisquare test for the categorical variables. To test the diagnostic accuracy of the strain parameters, we performed a receiver-operating characteristic analysis to determine the cut-off value of the peak systolic strain parameters that best differentiated patients with significant coronary stenosis from those without significant coronary stenosis. A p<0.05 was considered to indicate significance.
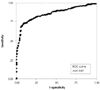
The major demographic and clinical characteristics of SA and ACS are given in Table 1. The peak systolic strains in the normal coronary group were relatively homogenous throughout the left ventricle (LV), they were higher in the base and lower in the apex, but the differences were not significant (Fig. 1). However, the peak systolic strains in the patients with significant coronary artery disease showed a marked heterogeneous pattern (Figs. 2 and 3) and this strain was significantly decreased in the ischemic segments as compared with the corresponding nonischemic segments (Table 2). Of the 179 SA patients, 74 had >70% stenosis (ischemic-SA) and 105 had normal coronary anatomy or 50% stenosis (normal-SA). Of the 74 patients in the ischemic-SA group, 23 patients (31%) showed a homogeneous pattern of peak systolic strain throughout the wall (strain negative) and 51 patients (69%) showed marked heterogeneity of strain (strain positive). Of the ischemic-SA group, 1-vessel disease was present in 50 patients, 2-vessel disease was present in 17 and 3-vessel disease was present in 7 patients. There was significant LAD artery stenosis in 49 patients, LCx stenosis was present in 27 and RCA stenosis was present in 30 patients. Multi-vessel disease was present in 7 patients of the 23 ischemic-SA patients with strain negative, and 21 patients of the 25 normal-SA patients with strain positive showed decreased apical strain. Of the 94 ACS patients, 67 had >70% stenosis (ischemic-ACS), and 27 had normal coronary anatomy or 50% stenosis (normal-ACS). Of the 67 patients in the ischemic-ACS group, 14 patients (21%) were determined to be strain negative, and 53 patients (79%) were determined to be strain positive. Of the ischemic-ACS group, 1-vessel disease was present in 28 patients, 2-vessel disease was present in 24 and 3-vessel disease was present in 15 patients. There was significant LAD artery stenosis in 38 patients, LCx artery stenosis in 17 patients and RCA stenosis in 22 patients. Multi-vessel disease was present in 11 patients of the 14 ischemic-ACS patients with strain negative, and 5 of the 9 normal-ACS patients with strain positive showed reduced apical strain (Table 3). Of these 4,914 segments, 157 segments (4%) were excluded from analysis due to an un-interpretable signal. Receiver-operating characteristic (ROC) analysis of the peak systolic strain (Fig. 4) yielded optimal cut-off values of -5.7 for the prediction of 70% coronary stenosis. The sensitivity and specificity of the peak systolic strain (values below the cutoff) were 71% and 93%, respectively. The ROC-area of the peak systolic strain for the LAD territory was 0.79 (95% CI 0.72-0.84); this was 0.87 (95% CI 0.79-0.91) for the LCx territory and 0.89 (95% CI 0.79-0.93) for the RCA territory.
The interobserver and intraobserver variability was tested with performing independent analysis by two independent observers and by repeated measurement of these segments on another occasion by one of the same observers. The interobserver variability was less than 20% and the intraobserver variability was 12%.
Performing conventional echocardiography for detecting ischemia-related systolic abnormalities involves visually estimating the changes of wall thickening in circular muscle. However, because it has been reported that regional mechanical events occur every 90 ms and postsystolic thickening (PST) happens every 50-60 ms, visual estimation has considerable limitations. So, techniques that quantify regional mechanics are being increasingly investigated as a means of objectively identifying myocardial ischemia. The concept of assessing myocardial stiffness by using a measure of deformation (i.e., strain) was described in 1973 by Mirsky and Parmley.11) The physical definition of strain is the relative change in length of a material related to its original length. The strain rate is the temporal derivative of the strain and so it expresses the local dynamics of myocardial performance. It can be mathematically shown that the strain rate is equivalent to the spatial gradient of velocities.12) Unlike tissue velocity imaging, strain imaging provides additional information, that is, a measure of a local instantaneous rate of myocardial compression or expansion, which is independent of cardiac translation. Moreover, the radial peak systolic strain of normal myocardium is linearly correlated with the M-mode ejection fraction, which is calculated with the Teichholz equation.13) The longitudinal systolic strain/rate has been shown to be linearly correlated with the maximal value of the first LV pressure time derivative and also with the peak elastance, which are both global measures of LV systolic function and contractility.14)15) Similar to tissue velocity imaging, strain echocardiographic imaging can be accomplished in real time, thus facilitating its clinical feasibility.12) The normal values for LV longitudinal shortening16) correspond well with our measurements: 19% for the average peak systolic strain verus 16.4% in our study. The slightly lower values of strain in our study would be due to the larger proportion of patients with hypertension and diabetes mellitus in our enrolled patients; and these diseases are known to cause abnormalities in tissue velocities. As the moving base descends toward the stationary apex, the tissue velocities have to decrease from the base to the apex.16)17) For the strain that's due to TDI, the myocardial displacement in our study also showed a base-to-apex gradient. However, according to previous studies,18)19) systolic strain is constant throughout the wall. Myocardial strain is relatively independent of translational motion and other through-plane motion effects, and it should be relatively homogeneous throughout the normal LV myocardium. As opposed to normal hearts, the LV of the ischemic heart in our study was characterized by marked heterogeneity of myocardial systolic strain, and tissue Doppler strain imaging demonstrated reduced shortening or stretching in the interrupted vessel territories. This was in contrast to the nonischemic region, where near-normal shortening was observed. It is known that the systolic strain showed the best correlation with conventional wall motion analysis, although the overall correlation is rather modest. From animal experiments, the regional strain values have been validated to correlate with those obtained from performing sonomicrometry in acute coronary ischemia.13) Reduced systolic strain appears earlier and so it is more sensitive than determining the presence of Doppler tissue velocity abnormality and arriving at a semiquantitative visual wall motion score in patients with acute ischemia.21) Furthermore, in both normal human and stunned porcine myocardium, the dobutamine-induced increase in systolic strain preceded the increase in LV systolic wall thickening.22) One of the main limitations of stress echocardiography is the length of the examination and the various thresholds of interpretation that directly influence its cost-effectiveness and clinical feasibility. The present study suggests that examination of systolic deformation with strain echocardiography may have significant diagnostic benefit for patients who have with chest pain even at rest.
This study has several limitations. First, several factors affect diagnostic accuracy, such as false positives and false negatives. The wall motion of the heart is more dynamic at the base than at the apical segment physiologically, and tissue Doppler-derived strain echocardiography is strongly angle dependent, and evaluation of the apex, which is a common site of ischemia, was difficult because of angulation issues. Therefore, further study with exclusion of the apical segments and comparison with the analysis of 2-dimensional strain that is not based on TDI is needed. Further, we did not take into account the salvage effect of reperfusion that results in normally functioning segments within the affected areas, so in the situation with well-developed collateral circulation such as multi-vessel disease or chronic total occlusion, the systolic deformation might be synchronous. Second, this study was not blinded; and some analysis of the strain parameters might be dependent on the clinical information. Therefore, further large-scale, blinded studies are required. Third, as was mentioned, the values of the strain for the patients with stenosis more than 50% and under 70% were not analyzed to get a more unambiguous result, and information about coronary lesion using intravascular ultrasound (IVUS) and myocardial perfusion scan was not available. Finally, among several strain parameters, we analyzed only the peak systolic strain because of signal noise, so the strain rate values were not compared with peak systolic strain and the coronary angiography results. In this study, our primary intention was to examine the relationship between regional systolic deformation and significant coronary disease at rest. To the best of our knowledge, this study is the first to compare the diagnostic value of strain echocardiography with coronary angiography in patients with suspected CAD in such a large group (n=273) and in a manner that was not confounded by referral bias.
In conclusion, we investigated whether analysis of regional deformation (strain) could identify regions subtended by significantly diseased coronary arteries in patients with or without active symptoms at rest, and our data demonstrated that abnormal regional systolic strain at rest might be associated with significant coronary stenosis.
Figures and Tables
Fig. 1
Strain echocardiography in a patient with normal angiography shows a relatively homogeneous pattern of peak systolic strains throughout the LV in the apical 4-chamber view (A), the apical 2-chamber view (B) and the apical 3-chamber view (C). LV: left ventricle.

Fig. 2
Strain echocardiography in a patient with significant left anterior descending coronary artery stenosis (A) shows a marked heterogeneous pattern of peak systolic strains throughout the LV in the apical 4-chamber view (B), the apical 2-chamber view (C) and the apical 3-chamber view (D). LV: left ventricle.
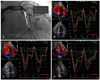
Fig. 3
Strain echocardiography in a patient with significant left anterior descending coronary artery stenosis (A) and right coronary artery stenosis (B) shows a marked heterogeneous pattern of peak systolic strains throughout the LV in the apical 3-chamber view (C) and the apical 2-chamber view (D). LV: left ventricle.
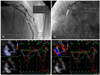
Fig. 4
Receiver-operating characteristic curves for peak systolic strain. AUC: area under the curve, ROC: receiver operating characteristics.
References
1. Hoffman R, Lethen H, Marwick T, et al. Analysis of interinstitutional observer agreement in interpretation of dobutamine stress echocardiograms. J Am Coll Cardiol. 1996. 27:330–336.
2. Kvitting JP, Wigstrom L, Strotmann JM, Sutherland GR. How accurate is visual assessment of synchronicity in myocardial motion?: an in vitro study with computer-simulated regional delay in myocardial motion: clinical implications forrest and stress echocardiography studies. J Am Soc Echocardiogr. 1999. 12:698–705.
3. Ehring T, Heusch G. Left ventricular asynchrony: an indicator of regional myocardial dysfunction. Am Heart J. 1990. 120:1047–1057.
4. Osakada G, Hess OM, Gallagher KP, Kemper WS, Ross J. End-systolic dimension-wall thickness relations during myocardial ischemia in conscious dogs: a new approach for defining regional function. Am J Cardiol. 1983. 51:1750–1758.
5. Wiegner AW, Allen GJ, Bing OH. Weak and strong myocardium in series: implications for segmental dysfunction. Am J Physiol. 1978. 235:H776–H783.
6. Leone BJ, Norris RM, Safwat A, Foex P, Ryder WA. Effects of progressive myocardial ischaemia on systolic function, diastolic dysfunction, and load dependent relaxation. Cardiovasc Res. 1992. 26:422–429.
7. Derumeaux G, Ovize M, Loufoua J, et al. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation. 1998. 97:1970–1977.
8. Derumeaux G, Ovize M, Loufoua J, Pontier G, Andre-Fouet X, Cribier A. Assessment of nonuniformity of transmural myocardial velocities by color-coded tissue Doppler imaging: characterization of normal, ischemic, and stunned myocardium. Circulation. 2000. 101:1390–1395.
9. Armstrong G, Pasquet A, Fukamachi K, Cardon L, Olstad B, Marwick T. Use of peak systolic strain as an index of regional left ventricular function: comparison with tissue Doppler velocity during dobutamine stress and myocardial ischemia. J Am Soc Echocardiogr. 2000. 13:731–737.
10. Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth O. Myocardial strain by Doppler echocardiography: validation of a new method to quantify regional myocardial function. Circulation. 2000. 102:1158–1164.
11. Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res. 1973. 33:233–243.
12. Heimdal A, Stoylen A, Torp H, Skaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998. 11:1013–1019.
13. Tennant R, Wiggers CJ. The effect of coronary occlusion on myocardial contraction. Am J Physiol. 1935. 112:351–361.
14. Visser CA, David GK, Kan G, et al. Two-dimensional echocardiography during percutaneous transluminal coronary angioplasty. Am Heart J. 1986. 111:1035–1041.
15. Wohlgelernter D, Cleman M, Highman HA, et al. Regional myocardial dysfunction during coronary angioplasty: evaluation by two-dimensional echocardiography and 12 lead electrocardiography. J Am Coll Cardiol. 1986. 7:1245–1254.
16. Voigt JU, Arnold M, Karlsson M, et al. Assessment of regional longitudinal myocardial strain rate derived from Doppler myocardial imaging indexes in normal and infarcted myocardium. J Am Soc Echocardiogr. 2000. 13:588–598.
17. Kukulski T, Jamal F, D'Hooge J, Bijnens B, De Scheerder I, Sutherland G. Acute changes in systolic and diastolic events during clinical coronary angioplasty: a comparison of regional velocity, strain rate and strain measurement. J Am Soc Echocardiogr. 2002. 15:1–12.
18. Cho KI, Park JH, Park JR, et al. Assessment of left ventricular function in symptomatic patients with myocardial bridge using two-dimensional strain. Korean Circ J. 2006. 36:617–625.
19. Stoylen A, Heimdal A, Bjornstard K, et al. Strain rate imaging by ultrasonography in the diagnosis of coronary artery disease. J Am Soc Echocardiogr. 2000. 13:1053–1064.
20. Henein MY, O'Sullivan CH, Davies SW, Sigwart U, Gibson DG. Effects of acute coronary occlusion and previous ischemic injury on left ventricular wall motion in humans. Heart. 1997. 77:338–345.
21. Cho GY, Park WJ, Han SW, et al. Quantification of regional wall motion abnormality using myocardial strain in acute myocardial infarction. Korean Circ J. 2003. 33:583–589.
22. Jamal F, Kukulski T, Strottman JM, et al. Quantitation of the spectrum of changes in regional myocardial function during acute ischemia in closed chest pigs: an ultrasonic strain rate and strain study. J Am Soc Echocardiogr. 2001. 14:874–884.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download