Abstract
Background and Objectives
Myocardial ischemia-reperfusion (I/R) injury is one of the major causes of cardiac mortality. Curcumin, an active component extracted from turmeric in curry, inhibits inflammatory responses. This study was designed to investigate whether curcumin can exert beneficial effects on myocardial I/R injury.
Materials and Methods
Sprague-Dawley male rats received a normal diet or a curcumin diet (80 mg/kg/d) for one week, and I/R injury was induced by ligating the left anterior descending artery (LAD) for 30 min followed by release. After 24 hours, the myocardium was extracted to evaluate the myeloperoxidase (MPO) activity and the vascular cellular adhesion molecule (VCAM)-1 protein level. The apoptotic cardiomyocytes and neutrophils were counted and quantified by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining at 14 days after I/R.
Results
In the infarcted myocardium of the curcumin-fed rats, the MPO activity (32.9±2.2% of the control, p=0.001) and the VCAM-1 protein (28.7±2.9% of control, p=0.001) level were significantly attenuated. The number of neutrophils was lower in the curcumin-fed rats (57±12% of the control, p=0.024). A reduction of the apoptotic cardiomyocytes was also observed in the curcumin-fed I/R rats (36±9.2% of the control, p=0.032).
Conclusion
The cardioprotective effects of curcumin on an I/R injury rat model could include anti-inflammation activities and inhibition of apoptosis that occurred in the cardiomyocytes. Our findings suggest that curcumin has a positive contribution as a dietary supplement for the prevention of heart disease.
The myocardial damage that's due to the reperfusion of ischemic tissue is primarily caused by proinflammatory cytokines such as tumor necrosis factor (TNF)-α1) and reactive oxygen species (ROS). Infiltrating neutrophils play a crucial role for the production of TNF-α in myocardial ischemia-reperfusion (I/R) injury.2)
Recent studies have shown that accumulated neutrophils may be involved in the pathogenesis of cardiomyocyte apoptosis by releasing various cytokines.3) Upon activation, these neutrophils not only generate ROS, but they also secrete myeloperoxidase (MPO). Vascular cellular adhesion molecule (VCAM)-1 is associated with inflammatory responses, and it was reported to be expressed on the endothelial cells' surface and it mediated the adhesion of leukocytes to the vascular endothelium.4)5)
Medicinal plants have recently become a focus of interest because they may play key roles in treating ischemic heart disease. Curcumin (diferuloyl methane) is the yellow pigment of turmeric in curry, and it is derived from the rhizome of the plant Curcuma longa. Curcumin is widely used as spice and it has been known to have anti-inflammatory and anti-cancer properties.6)7)
In addition, curcumin has been reported to scavenge free radicals and to inhibit nitric oxide synthase activity and the lipoxygenase and cyclooxygenase activities that are involved in the inflammatory pathways.8-10) A body of accumulated evidence suggests that curcumin is a potential anti-inflammatory agent that could suppress the induction of cytokines, the recruitment of immune cells and the progression of tissue damage.11)
In this study, we examined whether curcumin contributes to controlling important factors that have roles in aggravating myocardial I/R-injury.
Curcumin and collagenase were purchased from Sigma, and the in situ cell death detection kit was purchased from Roche. The Anaerobag was purchased from BD Science. VCAM-1 antibody, nuclear factor (NF)-κB p65 antibody, IκBα antibody, rabbit-horse radish peroxidase conjugate and WesternBreeze were purchased from Santa Cruz. Alexa488 conjugate antibody was purchased from Invitrogen. The images were obtained from a LAS-3000 analyzer (Fuji, Japan).
Curcumin (80 mg/kg/d) was mixed with the regular diet of male Sprague-Dawley rats (200±10 g) and this food was given to the rats for 7 days. After 7 days feeding, I/R injury was produced in two groups of rats: the I/R group and the curcumin+I/R group. The rats were intubated under anesthesia (1 mL/kg ketamine and 10 mg/kg xylazine, intraperitoneal) and positive pressure ventilation was maintained using a rodent respirator. The heart was exposed via left thoracotomy and I/R was induced by suture occlusion of the left anterior descending artery (LAD) for 30 minutes, and this was followed by release. The heart was restored to its normal position, and the chest was closed. The heart was extracted to evaluate the MPO activity (n=3 each in the I/R and curcumin+I/R groups), the malondialdehyde (MDA) formation (n=3 each in the I/R and curcumin+I/R groups), the VCAM-1 expression and the NF-κB nuclear translocation, as assessed by Western blotting (n=3 each in the I/R and curcumin+I/R groups) 24 hour after I/R-injury, and the heart tissues were also used to perform echocardiography and histological study (n=10 each in the I/R and curcumin+I/R groups) 2 weeks after I/R-injury. All the procedures in this study were approved by the Chonnam National University Animal Care and Use Committee.
To detect neutrophil infiltration, the activated neutrophils-specific MPO activity was determined in the rats' heart tissues. The hearts from the rats that were subjected to 30 minutes of ischemia and 24 hours reperfusion were assessed for their myeloperoxidase activity. The heart tissue was washed with cold phosphate-buffered saline (PBS), it was homogenized in a buffer containing 0.5% hexadecyltrimethylammonium bromide and it was centrifuged for 30 minutes at 20,000 g to collect the supernatant as a cytosol fraction. The cytosol was allowed to react with a solution of 1.6 mM tetramethylbenzidine and 0.1 mM H2O2. The absorbance change rate was measured by performing spectrophotometry at 650 nm.12) To determine the presence of infiltrated neutrophils, myocardial tissue was taken from an infarcted area of the heart at 24 hours after reperfusion, embedded in paraffin and stained with hematoxylin and eosin.
Lipid peroxidation was measured from the MDA content formed in the I/R injured rat heart. A homogenate of tissue was mixed with 100 mM ethylenediaminetetraacetic acid (EDTA) and 1% thiobarbituric acid (TBA) prepared in 0.5 M NaOH, and this was kept on a hot block for 30 minutes. Butylated hydroxytoluene was added to stop the reaction and the absorbance of the supernatant was measured at 532 nm.
The infarct and non-infarct myocardium tissue specimens were washed with ice-cold PBS, chopped on ice and resuspended in lysis buffer (20 mM Tris-HCl, pH 7.4, 0.1 mM EDTA, 150 mM NaCl, 1 mM phenylmethylsulphonyl fluoride, 1 µg/mL leupeptin, 1 mM Na3VO4). After centrifugation, the supernatant was prepared as a protein extract. Each extract of the tissue was fractionated by electrophoresis on 10% acrylamide gels and the proteins were transferred onto a polyvinylidene fluoride (PVDF) followed by blotting with the indicated antibodies. The protein levels were determined using Western Breeze reagents and an Image Reader (LAS-3000 Imaging System, Fuji Photo Film).
To determine the nuclear translocation of NF-κB p65 in the cardiomyocytes, cultured rat neonatal cardiomyocytes were prepared as previously described.13)
The cardiomyocytes were incubated within a sealed Anaerobag for 5 hours, and this was followed by reoxygenation for 30 minutes. The cardiomyocytes were fixed for 10 minutes with 2% paraformaldehyde at room temperature, washed three times with PBS, then permeabilized for 10 minutes with 0.5% Triton X-100 in PBS, washed three times with PBS and then incubated for 10 minutes in 1% bovine serum albumin (BSA) in PBS to block the non-specific binding sites before labeling was done with NF-κB p65 antibody. The primary antibodies were applied for 1 hour at room temperature, and this was followed by incubation with Alexa Fluor 488 goat anti-rabbit. The cell nuclei were counterstained with 4'-6-diamidino-2-phenylindole (DAPI). Recording and analysis of the fluorescence signals were performed using ImagePro software 5.0 (Media Cybernetics, Inc. MD, USA).
Two weeks after LAD ligation, two-dimensional (2-D) echocardiography was performed on the rats with using a Sonos 5500 ultrasonograph with a 15-MHz transducer (Philips, Andover, Massachusetts, USA), and the operator was kept "blind" to the status of the rats' treatment groups. The rats were placed in a custommade cradle on a heated platform in the supine or left lateral decubitus position to facilitate performing echocardiography. For quantification of the left ventricular (LV) dimensions and wall thickness, we recorded 2-D clips and the M-mode images in a short axis view from the mid-left ventricle at the tips of the papillary muscles.
The end diastolic and systolic LV diameters and both the anterior and posterior wall thicknesses were measured on the M-mode images with using the leading-edge-to-leading-edge convention. The LV fractional shortening and mass were calculated from the LV wall thickness and diameter (LVD) in the 2-D short axis view as: [(LVDdiastole-LVDsystole)/LVDdiastole]×100 and the LV mass was determined with the formula [1.04×{(LVID+PWT+IVST)}³-LVID³]×0.8+0.6, where LVID is the left ventricular end-diastolic internal dimension and IVST is the interventricular septum thickness.
After surgery, the animals were sacrificed using an overdose of anaesthesia, and the hearts were removed and fixed with 10% buffered formalin for further analysis. The heart tissue was cut into transverse blocks and then embedded in paraffin and serial sections were cut and placed on slides. The degree of fibrosis was evaluated by optical microscopy with using Masson's trichrome (MT) stain, and the area of fibrosis was calculated using National Institute of Health (NIH) densitometry software.
The apoptosis was determined by using a terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) method according to the manufacturer's protocol.
The enzymatic activity of the MPO released from the neutrophils was assayed and the number of infiltrated neutrophils was counted 24 hours after I/R induction. There was no significant difference of MPO activity in the non-infarct region between the I/R group and the curcumin+I/R group (1±0.8 for the I/R group vs. 1.3±1.1 for the curcumin+I/R group, p>0.05, Fig. 1A). On the other hand, the MPO activity was significantly higher in the infarct tissue than that in the non-infarct tissue by 14-folds (14.36±1.2 for the I/R group vs. 4.73±1.5 for the curcumin+I/R group, p<0.05, Fig. 1A). For the curcumin+I/R group, the MPO activity was significantly reduced by 33% compared with the I/R group. In addition to the MPO activity, the number of infiltrated neutrophils was lower in the curcumin+I/R group than that in the I/R group (0.57±0.1 in the curcumin+I/R group vs. 1±0.2 in the I/R group, p<0.05, Fig. 1B). Lipid peroxidation of the myocardium was determined by detecting MDA, a by-product of lipid peroxidation. The MDA formed in the non-infarct region was not different between two groups (100±5.1 in the I/R group vs. 92.2±10.1 in the curcumin+I/R group, p>0.05, Fig. 1C). On the other hand, MDA was reduced in the curcumin+I/R group compared with that in the I/R group (119.5±12.3 in the I/R group vs. 102.4±7.2 in the curcumin+I/R group, p<0.05, Fig. 1C).
The protein level of VCAM-1 was assessed by Western blotting. VCAM-1 protein was increased in the infarcted myocardium in the I/R group, while it was lowered in the infracted myocardium in the curcumin+I/R group (Fig. 2A), which suggested that curcumin blocked the I/R-mediated increased VCAM-1 protein expression.
Nuclear NF-κB p65 was increased in the I/R group while it was reduced in the curcumin+I/R group (Fig. 2B).
To confirm the inhibitory effect of curcumin on NF-κB activation, immunocytochemistry was performed on the rat neonatal cardiomyocytes. The cardiomyocytes were insulted with hypoxia/reoxygenation. As seen in Fig. 2C, the NF-κB p65 stained with green fluorescence. In the normal cardiomyocytes, the NF-κB p65 was in the cytosol in a diffuse manner. The NF-κB p65 translocated to nucleus, which was also stained with DAPI in the hypoxia/reoxygenated cardiomyocytes, where it abided in the perinuclear cytosol in the curcumin+hypoxia/reoxygenated cardiomyocytes.
Echocardiograms were performed to determine whether curcumin is capable of preserving the LV performance in post-myocardial infarction hearts. Echocardiography was performed by the M-mode at 2 weeks after I/R induction, and the left ventricular end-diastolic dimension (LVDd), the left ventricular diameter at end systole (LVDs), the interventricular septum thickness (LVS), the left ventricular posterior wall thickness (PWT), and the fractional shortening (FS) were all calculated. The representative M-mode echocardiograms are shown in Fig. 3 and the echocardiographic findings such as the IVS, LVDd, LVDs, PWT and FS are summarized in Table 1. Among them, the LVDs and FS showed significant deterioration in the I/R group compared with the normal group; in the curcumin+I/R group, both the LVDs and FS were significantly recovered compared with the I/R group (Fig. 3).
The area of fibrosis in each myocardial region was measured from the corresponding optical microscope picture of the Massaon's trichrome staining. The blue color represents the collagen deposits and quantitative image analysis revealed the marked decrease of fibrosis in the curcumin+I/R group compared with the I/R group (26.2±2.8% in the I/R group vs. 7.13±1.7% in the curcumin+I/R group, p<0.05, Fig. 4A). In addition, apoptosis was measured by TUNEL staining, and the number of positively stained cells was counted.
The apoptotic cell death was significantly reduced in the curcumin+I/R group (1.00±0.2 in the I/R group vs. 0.36±0.1 in the curcumin+I/R group, p<0.05, Fig. 4B).
In previous studies, curcumin has been reported to have a ROS scavenging property in addition to its anti-inflammatory and anti-cancer effects.10)11) Curcumin inhibited the NF-κB activation, which may participate with various stimuli such as oxidative stress, cytokines and hypoxic injury. Extensive research has recently shown that curcumin prevented oxidative stress-induced cellular injury by inhibition of NF-κB in steatohepatitis mice, intestine epithelial cells and microglial cells.14-16)
This study shows that I/R-induced myocardial damage such as neutrophil infiltration, fibrosis and apoptosis was attenuated in the curcumin-fed I/R rat model.
Vascular adhesion molecules play a primary role in these inflammatory processes.17) VCAM-1 is responsible for monocyte adhesion and increased vascular inflammation, and it is known to mediate transmigration of leukocytes to the endothelium and this leads to endothelial damage. In another reperfusion model, up-regulation of adhesion molecules on the surface of the airway epithelium may play a key role in the recruitment of inflammatory cells.18)
Reperfusion injury produces irreversible lipid peroxidation by providing oxygen that reacts with xanthene oxidase and hypoxanthine to produce highly toxic hydroxyl radicals in the presence of iron.19) Curcumin can scavenge free radicals, and it can block the tyrosine kinase enzyme activity that's responsible for apoptosis in renal epithelial cells and this tyrosine kinase enzyme activity is triggered by oxidative stress.20) Furthermore, curcumin could reduce the ongoing reperfusion injury that's mediated through inflammatory responses by interfering with NF-κB activation, which is critical in the regulation of transcription of pro-inflammatory related genes.8)21)22)
Inflammation contributes to many cardiovascular events, including I/R injury. In this study, I/R significantly increased the protein level of VCAM-1 and the level of neutrophil infiltration. Accumulated neutrophils may be involved in the pathogenesis of cardiomyocyte apoptosis by releasing various cytokines and oxidant species.3) The prior administration of curcumin was found to significantly lower the I/R-induced elevation seen at different levels of MPO activity, and it lowered the VCAM-1 expression, apoptotic cell death and LV dilation, and it decreased the factional shortening and cardiac fibrosis. Thus, curcumin may indirectly protect heart tissue from reperfusion injury. Although the exact mechanism by which curcumin protects against I/R injury in our rat model was not determined, it is suggested to include curcumin's free radical scavenging properties and its NF-κB inhibitory effect.
This study has several limitations. First, various studies that have employed animal models23)24) or human25)26) showed that curcumin is safe even at very high doses. Yet the bioavailability of curcumin is low outside of the gastro-intestinal tract,27) and absorption of curcumin in the body may occur both by gavage and intraperitoneal routes with different pharmacokinetic properties.28) In this study, the curcumin intake dosage was determined by referring to previous reports.27-29) The curcumin level was previously reported to be quantified by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS)30) and the curcumin plasma concentration of the curcumin-fed rats was not confirmed in our study due to technical difficulties. Second, although our data was statistical significance, the number of rats in this study should have been higher to prove the therapeutic effect of curcumin. Third, the difference of the infarct size relative to its area at risk in both the I/R group and the curcumin+I/R group should be estimated.
In this work, we studied whether curcumin could protect against I/R injury, and we checked the possible mechanisms that may have important roles. VCAM-1, oxidative stress and apoptosis were previously reported to be factors that mediate cardiac I/R injury. The responses to reperfusion implicate that oxidative stress and the apoptotic pathway are involved in injury to various types of heart cells, and curcumin may blunt these responses. Our data indicates that curcumin contributes to the attenuation of I/R injury by repressing the inflammatory and apoptotic pathways in the myocardium.
Figures and Tables
 | Fig. 1Myeloperoxidase (MPO) activity and malondialdehyde (MDA) formation were assessed at 24 hours after inducing I/R, and the neutrophils were counted at 2 weeks after inducing I/R. A: the MPO activity in the infarct region was significantly decreased in the curcumin+I/R group compared with that in the control group. B: the number of infiltrated neutrophils in the infarct region was reduced in the curcumin+I/R group compared with the I/R group. C: the level of MDA in the infarct region was lower in the curcumin+I/R group. *p<0.05, values are means±S.Ds. NI: non-infarct, I: infarct, I/R: ischemia-reperfusion, Cur: curcumin. |
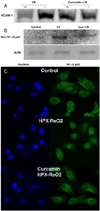 | Fig. 2The expression of VCAM-1 and the activation of NF-κB were examined. A: the level of VCAM-1 protein in the heart tissue 24 h after I/R injury was determined by Western blotting. The expression of VCAM-1 protein in the infarct region was attenuated in the curcumin+I/R group. B: the activation of NF-κB 24 h after I/R injury was determined by Western blotting by using NF-κB p65 subunit antibody. Activated NF-κB p65 was translocated to the nuclear fraction from the cytosol fraction. In the I/R group, nuclear factor (NF)-κB p65 was increased in the nuclear fraction (nuc) of the infarct region, while this was reduced in the curcumin+I/R group. C: immunocytochemistry was performed on the rat neonatal cardiomyocytes. Nuclear factor (NF)-κB p65 was stained with Alexa488 (green) and the nucleus was stained with DAPI (blue). In the normal cardiomyocytes, NF-κB p65 was located in the cytosol in a diffuse manner. NF-κB p65 was translocated to the nucleus in the hypoxia/reoxygenated cardiomyocytes, where it abided in the perinuclear cytosol in the curcumin+hypoxia/reoxygenated cardiomyocytes. NI: non-infarct, I: infarct, I/R: ischemia-reperfusion, Cur: curcumin, VCAM: vascular cellular adhesion molecule, DAPI: 4'-6-diamidino-2-phenylindole. |
 | Fig. 3M-mode echocardiography at 2 weeks after I/R. Fractional shortening (%) was significantly preserved in the curcumin+I/R group compared with that in the control group. *p<0.05, values are means±S.Ds. I/R: ischemia-reperfusion, Cur: curcumin. |
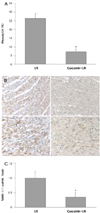 | Fig. 4Curcumin reduced the cardiac fibrosis and a poptosis. A: cardiac fibrosis was determined by Masson's trichrome staining. Fibrosis in the infarct region was significantly decreased in the curcumin+I/R group. B: apoptosis was assessed by TUNEL staining. C: the number of apoptotic cells (brown stained) in the infarct region was lower in the curcumin+I/R group than that in the I/R group (original magnification, 200×). *p<0.05, values are means±S.Ds. TUNEL: transferasemediated dUTP nick end labelihg, I/R: ischemia-reperfusion, Cur: curcumin. |
Acknowledgments
This work was supported by the Stem Cell Research Program funded by the Ministry of Science and Technology (MOST), Republic of Korea (M10641450001-06N4145-0011).
References
1. Shames BD, Barton HH, Reznikov LL, et al. Ischemia alone is sufficient to induce TNF-alpha mRNA and peptide in the myocardium. Shock. 2002. 17:114–119.
2. Brown JM, Anderson BO, Repine JE, et al. Neutrophils contribute to TNF induced myocardial tolerance to ischaemia. J Mol Cell Cardiol. 1992. 24:485–495.
3. Nakamura M, Wang NP, Zhao ZQ, et al. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. 2000. 45:661–670.
4. Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994. 84:2068–2101.
5. Cook-Mills JM. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol Immunol. 2002. 39:499–508.
6. Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005. 41:1955–1968.
7. Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991. 57:1–7.
8. Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995. 206:533–540.
9. Kang G, Kong PJ, Yuh YJ, et al. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci. 2004. 94:325–328.
10. Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995. 94:79–83.
11. Lin JK. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by Curcumin. Arch Pharm Res. 2004. 27:683–692.
12. Liu X, Xie W, Liu P, et al. Mechanism of the cardioprotection of rhEPO pretreatment on suppressing the inflammatory response in ischemia-reperfusion. Life Sci. 2006. 78:2255–2264.
13. Nitobe J, Yamaguchi S, Okuyama M, et al. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc Res. 2003. 57:119–128.
14. Leclercq IA, Farrell GC, Sempoux C, dela Pena A, Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004. 41:926–934.
15. Ma TY, Iwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kapp B activation. Am J Physiol Gastrointest Liver Physiol. 2004. 286:G367–G376.
16. Kim YS, Jhon DY, Lee KY. Involvement of ROS and JNK1 in selenite-induced apoptosis in Chang liver cells. Exp Mol Med. 2004. 36:157–164.
17. Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994. 8:504–512.
18. Georas SN, Liu MC, Newman W, Beall LD, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial cell activation accompany the recruitment of human granulocytes to the lung after segmental antigen challenge. Am J Respir Cell Mol Biol. 1992. 7:261–269.
19. Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988. 255:H1269–H1275.
20. Hagar H, Ueda N, Shah SV. Tyrosine phosphorylation in DNA damage and cell death in hypoxic injury to LLC-PK1 cells. Kidney Int. 1997. 51:1747–1753.
21. Kim YS, Ahn Y, Hong MH, et al. Curcumin attenuates nuclear factor-kapp B, c-Jun N-terminal kinase and p 38 in tumor necrosis factor-alpha-stimulated human endothelial cells. Korean Circ J. 2006. 36:482–489.
22. Ahn Y, Kim YS, Jeong MH. The role of nuclear factor kappa B activation in atherosclerosis and ischemic cardiac injury. Korean Circ J. 2006. 36:245–251.
23. Shankar TN, Shantha NV, Ramesh HP, Murthy IA, Murthy VS. Toxicity studies on turmeric (Curcuma longa): acute toxicity studies in rats, guineapigs & monkeys. Indian J Exp Biol. 1980. 18:73–75.
24. Qureshi S, Shah AH, Ageel AM. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Med. 1992. 58:124–127.
25. Lao CD, Demierre MF, Sondak VK. Targeting events in melanoma carcinogenesis for the prevention of melanoma. Expert Rev Anticancer Ther. 2006. 6:1559–1568.
26. Lao CD, Ruffin MT 4th, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006. 6:10.
27. Howells LM, Moiseeva EP, Neal CP, et al. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin. 2007. 28:1274–1304.
28. Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999. 27:486–494.
29. Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004. 74:969–985.
30. Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006. 40:720–727.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download