Abstract
Background and Objectives
Neointimal hyperplasia, which was caused by smooth muscle cell proliferation, was noted to occur after performing percutaneous coronary intervention. Phosphodiesterase type 5 (PDE5) inhibitor has been shown to inhibit smooth muscle cell proliferation. Udenafil is one of the PDE5 inhibitors, and it is also expected to inhibit smooth muscle cell proliferation and reduce neointimal hyperplasia. We investigated the effect of udenafil on the smooth muscle cell proliferation and neointimal hyperplasia that occurs after balloon injury in the carotid arteries of rats.
Materials and Methods
Smooth muscle cells were treated with 1 mM, 100 µM, 10 µM, 1 µM and 100 nM concentrations of udenafil. The viability of the smooth muscle cells was evaluated by MTT assay. The carotid arteries of rats were injured with a balloon catheter. Udenafil (100 µM, 10 µM and 1 µM) was applied on the carotid artery adventitia after balloon injury. At 21 days after treatment, the carotid arteries were harvested and stained with H & E. The neointima and media area were measured with a computerized image analysis program.
Figures and Tables
Fig. 1
MTT assay for the cell viability with using different udenafil concentrations. Quantification of HASMC proliferation was performed with MTT assay in presence of different concentrations of udenafil. *Vs control, †Vs 10 µM, ‡Vs 1 µM, §Vs 100 nM, p<0.05. HASMC: humen artery vascular smooth muscle cell.

Fig. 2
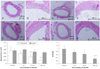
Histology analysis of the carotid artery. A: control, 100×. B: control, 400×. C: 1 uM udenafil, 100×. D: 1 uM udenafil, 400×. E: 10 uM udenafil, 100×. F: 10 uM udenafil, 400×. G: 100 uM udenafil, 100×. H: 100 uM udenafil, 400×. The bars of the graph represent the neointima area and media area and the neointima/media ratio. *Vs control, †Vs 10 uM, ‡Vs 1 uM, p<0.05. N/M: neointima/media.
References
1. Popma JJ, Califf RM, Topol EJ. Clinical trials of restenosis after coronary angioplasty. Circulation. 1991. 84:1426–1436.
2. Tahk SJ. Strategies for the prevention and treatment of intracoronary stent restenosis. Korean Circ J. 1997. 27:251–264.
3. Clowes AW, Clowes MM. Kinetics of cellular proliferation after arterial injury: II. inhibition of smooth muscle growth by heparin. Lab Invest. 1985. 52:611–616.
4. Park SJ, Kim HS, Yang HM, et al. Thalidomide as a potent inhibitor of neointimal hyperplasia after balloon injury in rat carotid artery. Korean Circ J. 2004. 34:346–355.
5. Tanaka H, Sukhova G, Schwartz D, Libby P. Proliferating arterial smooth muscle cells after balloon injury express TNF-alpha but interleukin-1 or basic fibroblastic growth factor. Arterioscler Thromb Vasc Biol. 1996. 16:12–18.
6. Clausell N, de Lima VC, Molossi S, et al. Expression of tumor necrosis factor alpha and accumulation of fibronectin in coronary artery restenotic lesions retrieved by atherectomy. Br Heart J. 1995. 73:534–539.
7. Linder V, Reidy MA. Expression of basic fibroblast growth factor and its receptor by smooth muscle cells and endothelium in injured rat arteries. Circ Res. 1993. 73:589–595.
8. Baek SH, March KL. Gene therapy for restenosis: getting nearer the heart of the matter. Circ Res. 1998. 82:295–305.
9. Cho MC, Kwak NJ, Piao H, et al. Effect of paclitaxel local delivery on neointimal formation after endothelial denudation of the rat carotid artery. Korean Circ J. 2000. 30:198–207.
10. Califf RM, Fortin DF, Frid DJ, et al. Restenosis after coronary angioplasty: an overview. J Am Coll Cardiol. 1991. 17:6 Suppl B. 2B–13B.
11. Seung KB. Drug eluting stent and percutaneous coronary intervention. Korean Circ J. 2003. 33:857–860.
12. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003. 349:1315–1323.
13. Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent. Circulation. 2004. 109:1942–1947.
14. Kalsi JS, Ralph DJ, Thomas P, et al. A nitric oxide-releasing PDE5 inhibitor relaxes human corpus cavernosum in the absence of endogenous nitric oxide. J Sex Med. 2005. 2:53–57.
15. Tantini B, Manes A, Fiumana E, et al. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol. 2005. 100:131–138.
16. Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989. 83:1774–1777.
17. Wharton J, Strange JW, Møller GM, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005. 172:105–113.
18. Fukumoto S, Koyama H, Hosoi M, et al. Distinct role of cAMP and cGMP in the cell cycle control of vascular smooth muscle cells. Circ Res. 1999. 85:985–991.
19. Chiche JD, Schlutsmeyer SM, Bloch DB, et al. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J Biol Chem. 1998. 273:34263–34271.
20. Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J. 1993. 7:328–338.
21. Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990. 87:5193–5197.
22. Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996. 78:225–230.
23. Mattsson EJ, Kohler TR, Vergel SM, Clowes AW. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997. 17:2245–2249.
24. Liu MW, Anderson PG, Luo JF, Roubin GS. Local delivery of ethanol inhibits intimal hyperplasia in pig coronary arteries after balloon injury. Circulation. 1997. 96:2295–2301.
25. Kwon JS, Park SS, Kim YG, et al. Perivascular delivery of paclitaxel with F-127 pluronic gel inhibits neointimal hyperplasia in a rat carotid artery injury model. Korean Circ J. 2005. 35:221–227.
26. Kim DW, Kwon JS, Kim YG, et al. Novel oral formulation of paclitaxel inhibits neointimal hyperplasia in a rat carotid artery injury model. Circulation. 2004. 109:1558–1563.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download