Abstract
Many patients with hypertrophic cardiomyopathy experience chest pain, and some of these patients are diagnosed with acute myocardial infarction. Acute myocardial infarction in the setting of hypertrophic cardiomyopathy can occur without coronary atherosclerosis. Although the exact pathophysiologic mechanism of this remains unclear, some pathologic studies have suggested that small vessel coronary artery disease in patients with hypertrophic cardiomyopathy may play a major role in producing myocardial ischemia. Small vessel disease can be suspected when the coronary angiogram shows patent epicardial coronary arteries with slow flow of the angiographic contrast medium. We report here on a case of hypertrophic cardiomyopathy that was complicated with acute myocardial infarction, and this induced catastrophic refractory ventricular tachycardia.
About 75% of the patients with hypertrophic cardiomyopathy (HCM) complain of angina and some cases are actually proven to be acute myocardial infarction (AMI).1) AMI is usually induced by coronary artery occlusion that's accompanied by atherosclerosis. However, several reports have documented the occurrence of AMI in the presence of normal or near normal coronary arteries. Although the pathogenesis is not completely understood, several hypotheses have been proposed.
We experienced a case of hypertrophic cardiomyopathy that was complicated with acute myocardial infarction, and this induced catastrophic refractory ventricular tachycardia. Large patent conducting coronary arteries with significant slow flow of the contrast medium were found on the coronary angiography of this patient.
A 58-year-old man was admitted to the hospital with chest pain that was associated with nausea and severe diaphoresis for 1 hour. His blood pressure was 60/40 mmHg and the heart rate 56 beats/min. The physical examination showed no abnormality of the chest and abdomen. He was diagnosed with hypertension 3 years ago, but he hadn't been taking his medicine regularly. The chest radiography demonstrated mild cardiomegaly and increased vascular markings on both hila. The ECG revealed ST segment elevation in leads V1-V3 and aVL, and ST segment depression in leads II, III and aVF (Fig. 1).
Resuscitation was achieved with intravenous fluids and inotropics, and we administered antiplatelet agents with heparin. The blood pressure was restored to 100/60 mmHg just before the coronary angiography. The initial cardiac enzymes were CPK: 7017 IU/L, CK-MB: > 300 ng/mL, Troponin-I: 3.7 ng/mL and myoglobin: 84 ng/mL.
The coronary angiography revealed markedly slow flow in the left anterior descending artery, but there was no significant coronary artery stenosis (Fig. 2). The TIMI frame count was 103 and the corrected TIMI frame count (CTFC) was 61 (normal range: ≤27). A CTFC is calculated by dividing the left anterior descending (LAD) artery's TIMI frame count by 1.7 to normalize for its longer length.2) Particularly, the flow was markedly slow below the mid-LAD. The TIMI frame count of the left circumflex artery (LCx) and the right coronary artery (RCA) was 57 and 40, respectively. Because the catheter tip was positioned in the LAD ostium, the LCx looked as if its flow rate was slower than its real flow rate. Although we could not perform additional coronary angiography due to the patient's poor condition, we estimated that the flow rate of the LCx was far faster than that of the LAD.
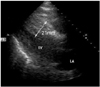
During the coronary angiography, the patient complained of sudden severe dyspnea and his systolic blood pressure decreased to 60 mmHg. He was intubated and an intraaortic balloon pump was inserted. The echocardiogram showed disproportionate hypertrophy of the interventricular septum compared to the left ventricular free wall (21 mm and 15 mm respectively), and hypokinesia of the anterior wall and an anteroseptum (Fig. 3).
Two hours after coronary angiography, the patient developed sustained ventricular tachycardia and his blood pressure went down to 60/40 mmHg (Fig. 4). Despite immediate and more than twenty times cardioversion ventricular tachycardia was not terminated and the patient expired.
Although patients with HCM frequently complain of chest pain (this found in about three-fourths of symptomatic patients), significant coronary artery disease was found in only 14-19% of the patients with HCM.3) Although various hypotheses have been proposed that explain the mechanism of myocardical infarction without significant epicardial coronary atherosclerosis, the true mechanism has yet to be elucidated. Increased myocardial mass, intramyocardial small vessel disease, inadequate vasodilator reserve, septal perforator artery compression, coronary artery spasm, elevated filling pressures related to impaired diastolic relaxation and increased oxygen demand due to dynamic outflow gradients have all been suggested as the mechanisms of myocardial ischemia.4)
Yet three pathologic reports make intramural coronary artery disease the most likely mechanism that induces acute myocardial infarction in patients with HCM. McReynolds et al.5) found that 24 of 32 patients with HCM had abnormalities in the intramural coronary arteries, and this consisted of medial hypertrophy and cellular intimal proliferation. In 1979, Maron et al.6) described the clinical and morphologic features of seven patients who suffered with both HCM and transmural myocardial infarction without significant atherosclerotic narrowing of the extramural coronary arteries. Marked abnormalities of the intramural coronary arteries were present in six of the seven patients. These abnormal vessels had thickened walls (due to intimal proliferation, medial hypertrophy or both) and narrowed lumens. In another report by Maron et al.,7) necropsies were performed on 48 patients who had suffered from HCM without atherosclerosis of the extramural coronary arteries and also on 68 control patients with either a normal heart or acquired heart disease. Of the 48 patients with HCM, 40 (84%) had abnormalities of the intramural coronary arteries, which was in contrast to the control group that had only 6 patients (9%) with abnormalities. Abnormal intramural coronary arteries were most common in the ventricular septum.
Slow coronary artery flow (SCAF) is an angiographic finding that's characterized by delayed distal vessel opacification in the absence of significant epicardial coronary disease and this is believed to represent coronary microvascular dysfunction. Abnormal intramural coronary arteries could be the cause of SCAF in patients with HCM.8)
Various arrhythmias are commonly observed in patients with hypertrophic cardiomyopathy and these may result in sudden cardiac death. Atrial fibrillation is the most common arrhythmia and this is observed in 20% of HCM patients, but ventricular tachyarrhythmias (VT/VF) are considered to be the most important cause of sudden cardiac death. Extensive disarray and fibrosis of the myocytes predispose to re-entrant ventricular arrhythmias. Various factors such as myocardial ischemia, systemic hypertension and supraventricular tachyarrhythmia etc. subsequently serve as triggers that induce malignant ventricular tachyarrhythmias.
In our patient, his abnormal intramural coronary artery that was represented by the slow coronary artery flow caused myocardial ischemia, and then the ischemia may have subsequently led to intractable VT.
Satoshi et al.9) reported that administering intracoronary nicorandil attenuated microvascular spasm, and Murakami et al.10) observed that intracoronary papaverine infusion in syndrome X patients increased the average peak velocity, it normalized the ST segment and it relieved chest pain. Although the pathophysiology of our case might not have been exactly the same as their patients, on hindsight, a better outcome may have been achieved with using these medications (nicorandil or papaverine).
Myocarditis can mimic acute myocardial infarction, and so it is suspected in patients who display the clinical and electrocardiographic evidence of acute coronary syndrome and they have a normal coronary angiogram. Yet in our case, the patient had no symptoms of any preceding viral or bacterial infection of the upper respiratory or gastrointestinal tract. There has been no previous report that myocarditis can be the cause of slow blood flow in a coronary artery.
Infiltrative restrictive cardiomyopathy also can mimic hypertrohic cardiomyopathy and it can cause sudden cardiac death. Especially in the setting of amyloidosis, asymmetric septal hypertrophy can be observed in some cases. But diffuse cardiac involvement that includes the atriums, valve leaflets and right ventricular wall is common, and pericardial effusion is frequently observed in patients suffering with cardiac amyloidosis. We did not observe these findings in our case, or the granular sparkling myocardium that is the most striking feature of cardiac amyloidosis. Further, our patient had no extracardiac manifestations such as renal failure, proteinuria, neuropathy and hepatomegaly. Although the electrocardiography did not show left ventricular hypertrophy in this patient, a normal ECG or an ECG without the criteria of LVH sometimes occurs in patients with HCM (6% and 35%, respectively).12) MRI or myocardial biopsy were needed to make a definite diagnosis, but we could not perform these methods due to the patient's very poor condition.
Although Hahm et al. have already reported on a case of HCM with myocardial infarction and a normal coronary arteriogram,11) this is the first documented combination of slow coronary artery flow and HCM that triggered VT in Korea.
Figures and Tables
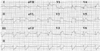 | Fig. 1The electrocardiogram on admission shows ST segment elevation in leads V1-V4 and aVL, and reciprocal ST depression in leads II, III and aVF. aVR: a ventricular right, aVL: a ventricular left, aVF: a ventricular function. |
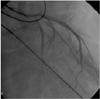 | Fig. 2The left coronary angiography revealed marked slow coronary artery flow, but no significant coronary artery stenosis. The corrected TIMI frame count was 61. TIMI: thrombolysis in myocardial infarction. |
References
1. Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 2005. 7th ed. Philadelphia: W.B. Saunders Company;1672.
2. Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996. 93:879–888.
3. Matsuura H. Hypertrophic cardiomyopathy complicated with ventricular aneurysm and myocardial necrosis. J Nucl Med. 1993. 34:2227–2235.
4. Lazzeroni E, Rolli A, Aurier E, Botti G. Clinical significance of coronary artery disease in hypertrophic cardiomyopathy. Am J Cardiol. 1992. 70:499–501.
5. Richard A, McReynolds RA, Roberts WC. The intramural coronary arteries in hypertrophic cardiomyopathy. Am J Cardiol. 1975. 35:154.
6. Maron BJ, Epstein SE, Roberts WC. Hypertrophic cardiomyopathy and transmural myocardial infarction without significant atherosclerosis of the extramural coronary arteries. Am J Cardiol. 1979. 43:1086–1102.
7. Maron BJ, Wolfson JK, Epstein SE, Robert WC. Intramural ("small vessel") coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986. 8:545–557.
8. Aksakal E, Yapici O, Yazici M, Yilmaz O, Sahin M. Apical hypertrophic cardiomyopathy: a case of slow flow in lad and malign ventricular arrhythmia. Int J Cardiovasc Imaging. 2005. 21:185–188.
9. Satoshi K, Ichiro I, Takuji K, et al. Intracoronary administration of nicorandil for the treatment of spontaneous microvascular spasm with ST segment elevation. Circ J. 2004. 68:267–269.
10. Murakami H, Urabe K, Nishimura M. Inappropriate microvascular constriction produced transient ST-segment elevation in patients with syndrome X. J Am Coll Cardiol. 1998. 32:1287–1294.
11. Hahm KB, Lee WK, Cho SY, Park KS, Jang YS, Chung NS. A case of hypertrophic cardiomyopathy with myocardial infarction and normal coronary arteriogram. Korean Circ J. 1986. 16:291–298.
12. Dumont CA, Monserrat L, Soler R, et al. Interpretation of electrocardiographic abnormalities in hypertrophic cardiomyopathy with cardiac magnetic resonance. Eur Heart J. 2006. 27:1725–1731.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download