Abstract
Background and Objectives
Adriamycin (doxorubicin, ADR) is a highly effective anti-neoplastic drug, but its clinical use is limited by its adverse side effects on the heart. Cardiotrophin (CT-1), a potent cardiac survival factor, is capable of inhibiting apoptosis in cardiac myocytes. The aim of this study was to investigate the cytoprotective effects of CT-1 against ADR-induced apoptosis in vitro.
Materials and Methods
We determined a reasonable ADR concentration for inducing cell death by utilizing a cell survival test performed in a dose-dependent manner. To determine the requirements for apoptosis in ADR-treated cardiac myocytes (H9c2 cells), we examined the effect of CT-1 on survival and apoptotic changes using a cell counting kit (CCK), RT-PCR, and Western blotting.
Results
In analyzing cell survival as determined by CCK, ADR-induced cell death was found to occur in a dose-dependent manner (50% death at 24 hours after 2 μM of ADR), and ADR was shown to decrease procaspase-3. On RT-PCR, expression of Bax-α mRNA increased and Bcl-2 decreased during the 24 hours after ADR treatment. Consequently, the ratio of Bax-α/Bcl-2 mRNA peaked at 24 hours after ADR treatment. In contrast, CT-1 effectively attenuated the ADR-induced cell death in a dose-dependent manner. The changes in Bax-α and Bcl-2 mRNA expression after ADR treatment were reversed by CT-1 (1 ng/mL) treatment. The protein levels of procaspase-3 decreased after ADR treatment, an effect which was reversed by CT-1 treatment. Akt phosphorylation was also increased by CT-1, demonstrating that CT-1 inhibited apoptosis induced by ADR.
Figures and Tables
 | Fig. 1Cell viability test performed at 24 hr after treatment with ADR (A), with or without CT-1 (B). ADR induced cell death in a dose dependent manner (Panel A) and CT-1 prevent ADR-induced cell death from the concentration of 1 ng/mL. Result presented in bar graph are mean±SEM of three experiments. *p<0.01 when compared with control cells. ADR: adriamycin, CT-1: cardiotrophin-1. |
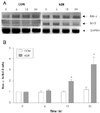 | Fig. 2Bax-α and Bcl-2 mRNA expression in H9c2 after ADR 2 μM treatment. Normalization relative to GAPDH was performed. Mean mRNA levels in untreated control cells are expressed as 1. *p<0.01 when compared to control cells. CON: untreated control cells, ADR: Adriamycin, GAPDH: Glyceride 3-phosphate dohydroqenase. |
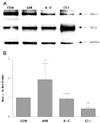 | Fig. 3Bax-α and Bcl-2 mRNA expression in H9c2 after ADR (2 μM) and CT-1 (1 ng/mL) treatment. Normalization relative to GAPDH was performed. The mean mRNA levels in untreated control cells are expressed as 1. *p<0.01 when compared with control cells. CON: untreated control cells, ADR: Adriamycin, A+C: Adriamycin 2 μM and Cardiotrophin-1 1 ng/mL, CT-1: cardiotrophin-1, GAPDH: Glyceride 3-phosphate dohydroqenase. |
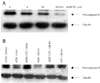 | Fig. 4ADR induces apoptosis in H9c2. A: H9c2 cells were treated with 10 μM ADR for various time and analyzed by western blotting using anti-caspase-3 antibody. B: H9c2 cells were treated with ADR (10 μM) and CT-1 (500 ng/mL). ADR induces apoptosis were decreased by CT-1 treatment. α-tubulin level was also examined as a loading control. ADR: adriamycin, CT-1: cardiotrophin-1, CON: untreated control cells. |
Acknowledgments
This work was supported by the Samsung Biomedical Research Institute grant, #SBRI C-A5-123-1.
References
1. Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998. 339:900–905.
2. Buzdar AU, Marcus C, Smith TL, Blumenschein GR. Early and delayed cardiotoxicity of doxorubicin. Cancer. 1985. 55:2761–2765.
3. Doroshow JH. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 1983. 43:460–472.
4. Rajagopalan S, Politi PM, Sinha BK, Myers CE. Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res. 1988. 48:4766–4769.
5. Papadopoulou LC, Theophilidis G, Thomopoulos GN, Tsiftsoglou AS. Structural and functional impairment of mitochondria in adriamycin-induced cardiomyopathy in mice: suppression of cytochrome c oxidase II gene expression. Biochem Pharmacol. 1999. 57:481–489.
6. Mijares A, Lopez JR. L-carnitine prevents increase in diastolic [Ca2+] induced by doxorubicin in cardiac cells. Eur J Pharmacol. 2001. 425:117–120.
7. Mimnaugh EG, Trush MA, Gram TE. Enhancement of rat heart microsomal lipid peroxidation following doxorubicin treatment in vivo. Cancer Treat Rep. 1983. 67:731–733.
8. Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977. 197:165–167.
9. Potmesil M, Israel M, Silber R. Two mechanisms of adriamycin-DNA interaction in L1210 cells. Biochem Pharmacol. 1984. 33:3137–3142.
10. Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984. 226:466–468.
11. Lampidis TJ, Johnson LV, Israel M. Effects of Adriamycin on rat heart cells in culture: increased accumulation and nucleoli fragmentation in cardiac muscle vs non-muscle cells. J Mol Cell Cardiol. 1981. 13:913–924.
12. Goldenberg GJ, Wang H, Blair GW. Resistance to adriamycin: relationship of cytotoxicity to drug uptake and DNA single- and double-strand breakage in cloned cell lines of adriamycin-sensitive and -resistant P388 leukemia. Cancer Res. 1986. 46:2978–2983.
13. Latchman DS. Cardiotrophin-1: a novel cytokine and its effects in the heart and other tissues. Pharmacol Ther. 2000. 85:29–37.
14. Sheng Z, Knowlton K, Chen J, Hoshijima M, Brown JH, Chien KR. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway: divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem. 1997. 272:5783–5791.
15. Kunisada K, Negoro S, Tone E, et al. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA. 2000. 97:315–319.
16. Lim BK, Choi JH, Jeon ES, et al. Virus receptor trap neutralizes coxackievirus in experimental murine viral myocarditis. Cardiovasc Res. 2006. 71:517–526.
17. Kim JM, Lim BK, Jeon ES, et al. TNFR-Fc fusion protein expressed by in vivo electroporation improves survival rates and myocardial injury in coxackievirus induced murine myocarditis. Biochem Biophys Res Commun. 2006. 344:765–771.
18. Wang L, Ma W, Markovic R, Chen JW, Wang PH. Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ Res. 1998. 83:516–522.
19. Jeon MH, Youn HJ, Lee JH. Adriamycin induced apoptosis of H9c2 cardiomycytes via a caspase-independent pathway. Korean Circ J. 2004. 34:76–83.
20. Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation. 2000. 102:572–578.
21. Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK. Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid Redox Signal. 2001. 3:135–145.
22. Park SW, Seo HS, Kim JW, et al. Effect of human Bcl-2 gene expression on the peripheral atherosclerotic lesions of apolipoprotein E-deficient mouse. Korean Circ J. 2005. 35:725–733.
23. Park CS, Youn HJ, Cho EJ, et al. Cardioprotective effect of IGF-1 in mouse with adriamycin-induced cardiomyopathy. Korean Circ J. 2002. 32:1116–1123.
24. Sung JD, Jeon ES. Pathogenesis of myocardial cell death and effect of cardiotrophin-1 in coxackieviral myocarditis. Korean J Cardiovasc Dis. 2002. 3:34–42.
25. Lopez N, Diez J, Fortuno MA. Characterization of the protective effects of cardiotrophin-1 against non-ischemic death stimuli in adult cardiomyocytes. Cytokine. 2005. 30:282–292.
26. Pennica D, King KL, Shaw KJ, et al. Expression cloning of cardiotrophin1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci USA. 1995. 92:1142–1146.
27. Kuwahara K, Saito Y, Kishimoto I, et al. Cardiotrophin-1 phosphorylates akt and BAD, and prolongs cell survival via a PI3K-dependent pathway in cardiac myocytes. J Mol Cell Cardiol. 2000. 32:1385–1394.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download