Introduction
A coronary artery aneurysm (CAA) is a dilatation that exceeds 1.5 times the diameter of the normal adjacent segment of the coronary artery,1) and its formation is a rare complication of stenting with bare-metal stents. Experimental studies suggest that drug-eluting stents may induce toxic effects on the vessel wall because of incomplete stent apposition, aneurysm formation, and the potential for stent thrombosis or vessel rupture.2)3) Coronary aneurysms have been described after the use of drug-eluting stents.2-7) Only one such case report has been published for Koreans.6) This report presents the first Korean case of a huge coronary aneurysm that was analyzed with using intravascular ultrasound (IVUS). We describe here a 62-year-old woman who developed a huge coronary aneurysm in the middle left anterior descending (LAD) artery and the first diagonal branch after receiving a sirolimus-eluting stent (CYPHER; Cordis, Miami Lakes, FL, USA). In contrast to the previous reports, she also had bare-metal stent sites with no sign of aneurysmal dilatation.
Case
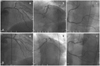
A non-hypertensive, non-diabetic, non-smoking 62-year-old woman was admitted to our coronary care unit, and she had been experiencing intermittent chest pain for 3 years. The chest pain was exercise-induced and it lasted for 1 min. On physical examination, she had an arterial pressure of 140/100 mmHg, a heart rate of 76 beats/min and a respiratory rate of 18 breaths/min. Cardiopulmonary auscultation was normal. The initial electrocardiogram revealed no abnormality and the cardiac enzymes were all within their normal ranges. The echocardiogram showed a good global left ventricle (LV) ejection fraction (EF) of 75%, with no regional wall motion abnormality of the left ventricle. Diagnostic coronary angiography revealed three-vessel disease, including a diffuse eccentric 72% diameter stenosis of the middle LAD artery, a diffuse eccentric 85% diameter stenosis of the first diagonal branch, a tubular eccentric 89% stenosis of the distal left circumflex (LCX) artery and a tubular eccentric 72% stenosis of the proximal posterior descending artery (PDA). The middle LAD lesion was predilated with an undersized balloon (2.5 mm), and a 3.0×23 mm sirolimus-eluting stent (CYPHER) was implanted with using an additional high-pressure balloon (2.75 mm). The first diagonal branch lesion was predilated with an undersized balloon (2.5 mm), and a 2.75×23 mm sirolimus-eluting stent (CYPHER) was implanted with using an additional high-pressure balloon (2.75 mm). The final kissing balloon inflation for the middle LAD and the first diagonal lesions was performed. We implanted a 2.5×20 mm bare-metal stent (Arthos; AMG, Westphalia, Germany) at the distal LCX lesion and a 2.75×16 mm cobalt-chromium stent (Arthos Pico) at the proximal PDA lesion (Fig. 1). The hospital course was unremarkable and she was discharged 3 days after the angiography procedure with aspirin 100 mg/day, clopidogrel 75 mg/day, simvastatin (a beta-blocker) and an angiotensin-converting enzyme inhibitor.
Six months later, she was admitted for routine coronary angiography after the previous implantation of multiple stents. The coronary angiogram was normal, with no sign of in-stent changes. She presented with a new episode of precordial pain 19 months after the previous implantation of the multiple stents. The subsequent coronary angiogram revealed a new 70% diameter stenosis lesion at the middle LAD, an aneurysmal dilation at the middle LAD stent lesion and in-stent restenosis at the ostium of the first diagonal branch with aneurysmal dilation. A 2.75×16 mm cobalt-chromium stent (Arthos Pico) was implanted at the new middle LAD lesion. The ostial lesion of the first diagonal branch was dilated with a 3.0×20 mm balloon.
Twenty-four months later, the coronary angiogram revealed progressive dilatation of the aneurysm (4 mm in depth) at the middle LAD stent lesion, with no sign of in-stent changes.
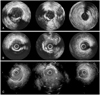
Forty-six months later, she presented to the emergency room with chest pain. The coronary angiogram revealed progressive dilatation of the aneurysms at both the middle LAD and the first diagonal branch stent lesions with no sign of in-stent changes (Fig. 2). The stent localization and aneurysmatic segment were analyzed using IVUS (Galaxy™; Boston Scientific, Reading, PA, USA), which revealed aneurysmal dilatation of the vessel outside the well expanded stent with a maximum aneurysm diameter of 12.4 mm (Figs. 3 and 4). No major neointimal thickening was observed. Considering her progressive disease state and the huge size of the aneurysm, she was advised to undergo further surgery.
Discussion
Randomized studies on the use of drug-eluting stents have demonstrated the inhibition of neointimal hyperplasia in the majority of patients.8)9) Moreover, gathering information on their long-term effect is extremely important with the increasing use of these stents.
CAAs are uncommon in patients undergoing angiography, with a reported incidence of 0.3 to 4.9%. An atherosclerotic etiology is the most common. Other etiologies include Kawasaki syndrome and congenital aneurysms, and aneurysms are now increasingly more common as the sequelae of coronary intervention.10)11)
The pathogenesis of coronary aneurysm formation has not been fully elucidated. The several possible mechanisms for coronary aneurysm formation include coronary dissection,12)13) the anti-inflammatory effects of the active drug,14) incomplete stent apposition,15) localized hypersensitivity vasculitis3) and the toxic effects of the carrier polymer.4)
Coronary aneurysms induced by balloon angioplasty may be caused by the use of oversized balloons or excessively high pressure. High-pressure balloon inflation is commonly used to optimize the expansion of coronary stents, and deep arterial injury is thought to be the main cause of aneurysm formation after coronary intervention. Injury or resection of the media may lead to gradual dilatation and thinning of the vessel wall. The reduced wall thickness and increased stress ultimately cause formation of the aneurysm. Dissection of the coronary artery during percutaneous balloon angioplasty is the main factor related to the occurrence of a CAA.12)13)
IVUS following stent placement may have the ability to detect the incomplete coverage or incomplete apposition of the stent to the arterial wall, and this may not be detected by plain angiography alone.
Rab et al.14) reported that aneurysm formation probably increased with the concomitant use of anti-inflammatory agents. Sirolimus acts specifically at the G1 phase of the cell cycle, and due to its early action on the cell cycle, sirolimus can block cell proliferation and so it has the potential to induce late vascular sequelae.9) In addition, sirolimus stimulates apoptosis and reduces inflammation. Therefore, drug-eluting stents can lead to the formation of coronary aneurysms.
Among the late postimplantation findings, incomplete apposition, which is defined as separation of the stent filaments from the intima, could be the mechanism responsible for aneurysms.15) Various hypotheses have been formulated to explain their origin, such as positive regional vascular remodeling, plaque regression, the late dissolution of the thrombotic material captured by the stent filaments, cell necrosis, apoptosis and an allergic reaction to sirolimus.16) It was recently reported that late stent malapposition occurred more commonly after drug-eluting stent implantation than after bare-metal stent implantation.17)18)
A case of fatal acute myocardial infarction due to late thrombosis of a CYPHER stent deployed 18 months earlier has been reported.3) The autopsy showed that the wall of the stented artery was dilated aneurysmally with an extensive inflammatory infiltrate involving the intima, media and adventitia. Based on the pathological evidence, local hypersensitivity vasculitis in response to the polymer of the CYPHER stent was regarded as the most likely mechanism.3)
Another concern is the possible toxic effects of the carrier polymer that is used as the drug platform. Numerous synthetic polymers have been applied to the metallic surface to serve as a matrix for the active drug.4)
Concern about the fate of coronary aneurysm formation after drug-eluting stent implantation has been raised because of the substantial complications such as stent thrombosis, acute myocardial infarction,19) vessel rupture and sudden death. Unfortunately, the optimal management for this condition remains controversial.
In our case, other causes may have been involved in addition to stenting with a drug-eluting stent. Considering the patient's progressive disease state and the huge size of the aneurysm, the patient was advised to undergo surgery.
In summary, we described here a case with delayed development of a giant coronary aneurysm after stent placement, and this occurred despite the excellent angiographic result.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download