Abstract
A 67-year-old male with stable angina was admitted to our cardiovascular center. He had neither any history of smoking, diabetes mellitus, hypertension, cerebrovascular accident nor family history of coronary artery disease. Coronary angiography showed a 90% tubular eccentric luminal narrowing at the mid left anterior descending artery (m-LAD). A sirolimus-eluting stent (SES) was implanted in the m-LAD. Coronary angiography performed after 9 months did not reveal restenosis or recurrent coronary artery disease. However, the patient returned to the emergency room with severe chest pain after 17 months. Coronary angiography showed severe diffuse vasospasm distal to the m-LAD stent site. After 20 days, vasospastic myocardial infarction developed. A zotarolimus-eluting stent with a phosphorylcholine polymer was implanted distal to the m-LAD stent. The zotarolimus-eluting stent was used because the polymer in the SES or sirolimus was considered a possible cause for the recurrent vasospasm. The patient had no further chest pain during the 9 months after zotarolimus-eluting stent implantation. We suspect that the polymer in the SES or sirolimus might have caused endothelial dysfunction and provoked the late vasospasm. Here, we describe this case of late recurrent vasospasm after SES implantation.
Sirolimus-eluting stents (SESs) have been shown to reduce the incidence of angiographic and clinical restenosis. 1-3) However, the antiproliferative properties of the currently available SESs delay vessel healing.4)5) Preliminary data suggest that SES is associated with endothelial dysfunction and impairment of endothelial vasomotor functions. Endothelial dysfunction and incomplete vascular healing may play a key role in the development of paradoxical vasoconstriction. Recent studies have reported on coronary vasomotor responses after SES implantation. Some investigators have concluded that implantation of an SES is associated with endothelial dysfunction in the proximal and distal regions of the treated coronary segment, whereas implantation of bare-metal stents does not produce any vascular response to exercise or acetylcholine infusion.6)7) In addition, Maekawa et al.8) using an acetylcholine test 6 months after the intervention, reported severe endothelial dysfunction distal to the implanted stent site.
The present report describes a case of late recurrent vasospasm 17 months post-SES implantation even after treatment with a calcium-channel blocker, nitrate, nicorandil, and an alpha-channel blocker. We successfully implanted a zotarolimus-eluting stent (Endeavor™, Medtronic Ireland, Ireland) in the vasospastic lesion distal to the segment containing the SES. The patient has remained asymptomatic during the 9 months after the implantation of the zotarolimus-eluting stent. We suspect that the late recurrent vasospasm was due to endothelial dysfunction associated with SES implantation.
A 67-year-old male with stable angina was admitted to our cardiovascular center. He had no risk factors such as diabetes mellitus, hypertension, hypercholesterolemia, alcohol, or smoking. Coronary angiography showed a 90% diffuse eccentric luminal narrowing at the mid left anterior descending artery (m-LAD). The m-LAD artery lesion was predilated with an undersized balloon (2.5 mm×20 mm at 8 ATM), and a sirolimus-eluting stent (3.0×28 mm at 12 ATM) was implanted (Fig. 1). The patient was discharged without any in-hospital complications.
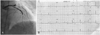
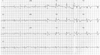
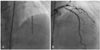
The patient underwent follow-up angiography at 9 months after stenting. The coronary angiography did not show restenosis or recurrence of coronary artery disease. The patient was asymptomatic and was treated with a β-blocker, aspirin, clopidogrel, nitrate, and atorvastatin. However, he returned to the emergency room with severe chest pain at 17 months after stenting; at this time, the cardiac enzymes were normal. Coronary angiography showed a severe diffuse vasospasm distal to the m-LAD stent site (Fig. 2). The vasospasm improved after treatment with intracoronary nitrate administration (Fig. 3). The patient was discharged with the prescription of a calcium-channel blocker, nitrate, nicorandil, alpha-channel blocker, aspirin, and clopidogrel. At 20 days after discharge, a vasospastic myocardial infarction developed. The levels of cardiac enzymes were markedly elevated (peak troponin-I, 60 ng/mL; peak CK-MB, 190 mg/L). ST segment elevation had developed in leads V1-V5 on electrocardiography (Fig. 4). A coronary angiography was performed, and it did not show significant stenosis or thrombosis and the stent was patent. We considered that the severe spasm, distal to the m-LAD stent site, caused the vasospastic myocardial infarction. Therefore, we decided to insert a zotarolimus-eluting stent with a phosphorylcholine polymer distal to the m-LAD stent to cover the vasospastic arterial segment. The non-erodible polymer in the SES might have caused the vasospasm (Fig. 5A). An excellent angiographic result was achieved (Fig. 5B), and the patient has remained asymptomatic during the 9 months after zotarolimus-eluting stent implantation. We suspect that the abnormal vasoconstriction in the arterial segment distal to the SES was due to a local effect of the sirolimus or non-erodible SES polymer on the endothelial function.
Sirolimus inhibits endothelial cell as well as smooth muscle cell proliferation by cell cycle arrest in the late G1 phase of cell division.4)5) This impairment of endothelial recovery after SES implantation may have adverse effects on the vasomotor function. Abnormal endothelial responses have been demonstrated 6 months after transluminal coronary angioplasty with SES. Fuke et al.7) used acetylcholine to demonstrate paradoxical vaoconstriction in peristent segments at 6 months after stent deployment. The paradoxical response of the stentimplanted arteries has been attributed to reduced nitric oxide bioavailability at the site of stenting. Acetylcholine dilated normal coronary artery segments by promoting the synthesis and release of nitric oxide in the endothelium.11) Contrastingly, in segments with endothelial damage, acetylcholine constricted the coronary arteries because of disturbed nitric oxide synthesis and release.12) Togni et al evaluated the endothelial function by studying the flow-mediated changes induced by exercise. They reported that exercise-in-duced coronary vasodilation in the peristent area was impaired in patients with SES, whereas it was not impaired after bare-metal stent implantation.6) However, there is no report on an abnormal vasomotor response at 6 months after implantation.
In this case, the vasospasm occurred approximately 17 months after stenting without any significant stimulation. The vasodilator response to nitroglycerin was maintained. The Cypher stent™ (Cordis, Johnson & Johnson, Miami Lakes, FL, USA) was designed to achieve more than 80% sirolimus elution by 30 days after implantation13) and 100% elution by 60 days.14) Therefore, the endothelial dysfunction 17 months after the SES implantation was not due to a direct effect of sirolimus. The relationship between adverse clinical events and abnormal vasomotor activity after stent implantation is speculative and remains to be confirmed. However, severe endothelial dysfunction in the absence of an obstructive coronary artery disease is associated with increased cardiac events.15)
A recent report has described severe multivessel paclitaxel-eluting stent implantation.9) Wheatcroft et al have described similar events immediately after SES implantation.10) However, the exact mechanism underlying coronary artery spasm after stent implantation is not known. Endothelial dysfunction after drug-eluting stent implantation may be caused by drugs (sirolimus, paclitaxel, etc) or the non-erodible polymer and hypersensitivity reactions.
Pharmacologic therapy remains the treatment of choice for vasospasm. Some reports have shown good results after coronary stenting in patients with refractory vasospastic angina.16-20) The decision to implant zotarolimus-eluting stent (Endeavor™, Medtronic, Ireland) in patients with vasospastic coronary arteries is based on several factors including recurrent angina refractory to aggressive drug therapy; documented, localized spasm at the site distal to the previous stent; and the occurrence vasospastic myocardial infarction. A non-erodible polymer is the platform used for SES stents that have been associated with endothelial dysfunction. Therefore, we used the zotarolimus-eluting stent with a biocompatible phosphorylcholine polymer.
In conclusion, using an SES has been associated with the potential risk of unfavorable alteration of coronary vasomotor activity. Abnormal coronary vasomotor activity may cause severe refractory vasospasm. We successfully managed a refractory coronary vasospasm due to endothelial dysfunction with a zotarolimus-eluting stent. A further study is required for the development of adequate prevention and treatment of endothelial dysfunction associated with stent implantation.
Figures and Tables
Fig. 1
Angiogram of the left anterior descending artery. It shows a diffuse long stenotic lesion before (A) and immediately after (B) sirolimus-eluting stent placement.
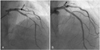
Fig. 2
Angiogram and electrocardiography after 17 months. Diffuse luminal narrowing distal to the m-LAD stent site (A) and electrocardiographic findings (B). m-LAD: mid left anterior descending artery.
References
1. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002. 346:1773–1780.
2. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003. 349:1315–1323.
3. Nakamura M, Wada M, Hara H, Kozuma K, Otsuka Y, Miyazaki S. Angiographic and clinical outcomes of a pharmacokinetic study of sirolimus-eluting stents: lesson from restenosis cases. Circ J. 2005. 69:1196–1201.
4. Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995. 76:412–417.
5. Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002. 8:128–135.
6. Togni M, Windecker S, Cocchia R, et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol. 2005. 46:231–236.
7. Fuke S, Maekawa K, Kawamoto K, et al. Impaired endothelial vasomotor function after sirolimus-eluting stent implantation. Circ J. 2007. 71:220–225.
8. Maekawa K, Kawamoto K, Fuke S, et al. Severe endothelial dysfunction after sirolimus-eluting stent implantation. Circulation. 2006. 113:e850–e851.
9. Kim JW, Park CG, Seo HS, Oh DJ. Delayed severe multivessel spasm and aborted sudden death after Taxus stent implantation. Heart. 2005. 91:e15.
10. Wheatcroft S, Byrne J, Thomas M, MacCarthy P. Life-threatening coronary artery spasm following sirolimus-eluting stent deployment. J Am Coll Cardiol. 2006. 47:1911–1912.
11. Takase B, Hamabe A, Satomura K, et al. Comparable prognostic value of vasodilator response to acetylcholine in brachial and coronary arteries for predicting long-term cardiovascular events in suspected coronary artery disease. Circ J. 2006. 70:49–56.
12. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986. 315:1046–1051.
13. Klugherz BD, Llanos G, Lieuallen W, et al. Twenty-eight-day efficacy and pharmacokinetics of the sirolimus-eluting stent. Coron Artery Dis. 2002. 13:183–188.
14. Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004. 109:701–705.
15. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000. 101:948–954.
16. Marti V, Ligero C, Garcia J, Kastanis P, Guindo J, Dominguez de Rozas JM. Stent implantation in variant angina refractory to medical treatment. Clin Cardiol. 2006. 29:530–533.
17. Gaspardone A, Tomai F, Versaci F, et al. Coronary artery stent placement in patients with variant angina refractory to medical treatment. Am J Cardiol. 1999. 84:96–98.
18. Nakamura T, Furukawa K, Uchiyama H, Seo Y, Okuda S, Ebizawa T. Stent placement for recurrent vasospastic angina resistant to medical treatment. Cathet Cardiovasc Diagn. 1997. 42:440–443.
19. Seung KB. Drug eluting stent and percutaneous coronary intervention. Korean Circ J. 2003. 33:857–860.
20. Kim JY, Yoon J. Aspirin and clopidogrel resistance in drug eluting stent era. Korean Circ J. 2007. 37:135–147.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download