Abstract
Background and Objectives
Coronary artery disease (CAD) in a transplanted heart has been a major cause of morbidity and mortality for the transplantation patients who survive more than 1 year. The incidence and characteristic pattern of coronary artery disease after heart transplantation in Koreans are not known. The aim of this study is to determine the incidence and characteristics of significant CAD and the coronary arterial remodeling pattern after heart transplantation by using intravascular ultrasound (IVUS).
Subjects and Methods
We evaluated a total of 101 consecutive patients who had been examined via serial (over one year interval) IVUS and coronary angiogram. The patients were divided into two groups according to the period of the serial IVUS follow-up. The post-transplant early period (EP) group (n=58) was defined when IVUS was performed within the first month and at one year after heart transplantation, and the post-transplant late period (LP) group (n=43) was defined when IVUS was performed after one year and subsequently over another one year interval.
Results
The CAD-free survival rates, as assessed by coronary angiogram, were 99% at 5 years, 89% at 7 years and 71% at 9 years. In the EP group, 17 patients (29%) had donor lesions and 8 patients (14%) had de novo lesions. For the donor lesions, the diffuse and concentric lesions were 12%, respectively, and the remodeling index was 2.3±6.5, which represents a positive remodeling pattern. For the de novo lesion, the diffuse lesions were 25%, the concentric lesions were 13% and the remodeling index was -2.5±4.9, which represent a negative remodeling pattern.
Heart transplantation is an established therapeutic option for advanced heart failure. The median survival period after heart transplantation is around 10 years, and the leading cause of death after one year for heart transplant recipients is transplant coronary artery disease (tCAD).1) Angiographic and IVUS studies have shown that the pathogenesis of tCAD is progressive intimal thickening. However, it is unclear whether arterial remodeling plays a significant role in the pathobiology of tCAD.2) In the reports from Western countries, the incidence of donor and de novo lesions in heart transplanted patients was 30-40%, respectively, with 50% of lesions being diffuse and 50% concentric. However, no information is currently available regarding the incidence and characteristics of tCAD after heart transplantation in Koreans. Therefore, we performed serial IVUS examinations to determine the incidence and characteristics of donor and de novo tCAD in transplanted hearts, as well as to compare the vascular change and remodeling patterns during the subsequent years after heart transplantation in Koreans.
One hundred seventeen patients underwent two or more serial coronary angiograms. We started the serial IVUS examinations for 101 patients from Nov, 1999 to Aug, 2005 at Asan Medical Center to evaluate transplant coronary artery disease. Of these, 58 patients underwent IVUS examinations both within the 1st month of transplantation and at 1st year after transplantation: these patients comprised the post-transplant early period (EP) group. Another 43 patients underwent the 1st IVUS examination at the 1st year after transplantation. These 43 patients comprised the posttransplant late period(LP) group.
Following performance of a diagnostic coronary angiogram, the patients were given intravenous heparin and intracoronary nitroglycerin. A 30-40 MHz, 3.2 or 2.6 F monorail ultrasound catheter(Galaxy II, 40 MHz; Boston Scientific, USA) connected to a dedicated scanner was advanced over an angioplasty guidewire. The most distal transducer location was documented by angiography, and this was followed by pulling back the ultrasound catheter, from distal to proximal, with using an automatic pullback system at 0.5 mm/second, with continuous recording on videotape. The left anterior descending artery was imaged from the distal to the proximal part. In the EP group(n=58), the initial IVUS was performed within 4 weeks after heart transplantation and the follow-up IVUS was performed at 12±1 months after transplantation. In the LP group(n=43), baseline and follow-up IVUS examinations were performed at 15±6 and 47±21 months after transplantation, respectively(Fig. 1).
Using the guiding catheter for calibrating the magnification and an online QCA system(ANCOR V2.0; Siemens, Germany), the target lesion with a minimal luminal diameter(MLD) was measured. QCA measurements of the MLD were performed after angiography, over a one-year period of follow-up, from the diastolic frames in a single matched view showing the smallest luminal diameter. Reference diameters were selected from user-defined segments that were proximal and distal to the lesion.
Measurements were performed according to the American College of Cardiology clinical expert consensus document on standards for the acquisition, measurement and reporting of IVUS studies.3) Measurements were taken of the external elastic membrane(EEM), lumen, plaque and media(P&M=EEM-lumen), and the intimal hyperplasia(IH=intima-lumen) cross-sectional areas(CSA) with using a commercially available program for computerized planimetry(TapeMeasure, Indec System). The site of maximal IH was identified at the baseline examination and this was used as reference for comparison in subsequent examinations.
A "Donor lesion" was defined as a site of maximal intimal thickness over 0.5 mm at the time of the baseline examination. A "de novo lesion" was defined as the maximum intimal thickness over 0.5 mm on follow-up at a site where the intimal thickness was below 0.3 mm at the time of the baseline examination(Fig. 2). A target lesion was defined as the most thickened intimal site. Diffuse lesion was defined as the lesion with a maximal intimal thickness over 0.5 mm and the total lesion length was ≥20 mm, and focal lesion was defined as the total lesion length was below 20 mm. A concentric lesion was defined as its distribution arc was ≥270°, and eccentric lesion was defined as its distribution arc was <270°(Fig. 3). A reference vessel site was defined as a site 20 mm from a target lesion. The remodeling index(RI), which is a quantitative measure of the extent and direction of remodeling, was calculated as the change in the vascular area at the lesion site divided by the change in the intimal area.4) An RI ≥1 indicated positive remodeling that adequately compensated(=1) or overcompensated(>1) for intimal growth, an index >0 and <1 indicated positive remodeling that was inadequate for intimal growth, and an RI ≤0 indicated negative remodeling with no compensation(=0) or "shrinkage"(<1) of the vessel(Fig. 4). The Intimal index(II) was calculated as(intimal area)/(lumen+intimal area). The eccentricity index was defined as the maximal intimal thickness divided by the minimal intimal thickness within the target lesion. Eccentric lesion was defined as the eccentricity index(EI)>3. Angiographically significant lesion was defined as the lesion showing a diameter stenosis more than 50%.5)
Means±SD were calculated for all the numerical data. Comparison of the data of the serial IVUS examinations was done using non-paired t tests. The coronary artery disease(CAD)-free survival analysis was performed with the Kaplan-Meier method for the heart transplantation patients and for those patients presenting with significant coronary lesions. A p<0.05 was considered significant. All analyses were performed using SPSS statistical software, version 11.0.
Table 1 summarizes the clinical data of the total patient population(n=101). The mean age of the donors was 28.5±9.3 years(range: 11-49 years) and the mean age of the recipients was 40.1±13.0 years(range: 15-65 years). Four percent of the patients had cytomegaloviral disease. The risk factors for coronary artery disease, diabetes mellitus, hypertension and hypercholesterolemia were founded in 19%, 21% and 17% of the patients, respectively.
From the baseline and follow-up IVUS data of the 101 patients, the plaque area(4.1±2.3 mm2 vs 5.2±2.5 mm2, respectively, p=0.004), lumen area(13.3±3.6 mm2 vs 12.2±3.2 mm2, respectively, p=0.004) and intimal index(23.7±12.5 vs 29.9±13.0, respectively, p=0.001) changed significantly, but the target vessel area (17.4±3.7 mm2 vs 17.4±3.4 mm2, respectively, p=0.92) did not(Table 2). The maximal intimal thickness of the target lesion in all patients increased significantly, from 0.45±0.24 mm at baseline to 0.55±0.24 mm at follow-up(p=0.001). The intimal index also increased significantly, from 0.24±0.13 at baseline to 0.30±0.14 at follow-up (p=0.001). Of these 101 patients, 67 patients(66%) showed significant intimal thickness(≥0.5 mm) at more than one year after transplantation. The distribution of these significant lesions were proximal segments in 42 patients (62%), middle segments in 18 patients(27%) and distal segments in seven patients(11%).
In the EP group, donor lesions were identified in 17 patients(29%) at baseline, 8 patients(14%) developed de novo lesions and 31 patients(57%) showed to be normal. For the donor lesions, 2(12%) were diffuse and concentric. 11 patients(65%) had lesions located in proximal segments, four(24%) had lesion in the mid segments and two(11%) had lesion in the distal segments. For the de novo lesions, two(25%) were diffuse and one(13%) was concentric. Six lesions(75%) were located in the proximal segments and two(25%) were located in the mid segments. Fifteen patients(83%) with donor lesions and six patients(75%) with de novo lesions had eccentric lesion. Calcification of target lesions was found in four patients(7%).
The IVUS data between the EP and LP groups showed that the change of the lumen area(1.01±1.55 mm2 vs 0.83±1.52 mm2, respectively, p=0.001) and the plaque area(-1.85±3.01 mm2 vs -0.16±2.44 mm2, respectively, p=0.001) were greater in the EP group than in the LP group, but the change of the intimal index(6.8±9.7% vs 3.6±7.2%, respectively, p=0.45) was not different (Table 3). The vessel area decreased in the EP group, but it increased in the LP group(-0.85±2.26 mm2 vs 0.67±3.05 mm2, respectively, p=0.001). The change of intimal thickness(0.14±0.22 mm vs 0.05±0.21 mm, respectively, p<0.001) was greater in the EP group than in the LP group. The RI was >1 in 11 patients(29%), ≤1 but >0 in five patients(13%), and ≤0 in 22 patients (58%) in the EP group, and >1 in 9 patients(23%), ≤1 but >0 in 13 patients(33%), and ≤0 in 17 patients (44%) in the LP group.
The areas of the proximal reference vessel and the lumen areas of the donor lesions were 19.8±4.2 and 19.3±3.6 mm2(p=0.28) respectively, at baseline and 15.2±4.1 and 14.5±3.5 mm2(p=0.43) respectively, at follow-up. The vessel area, lumen area and plaque area of the target lesions and II were not changed significantly between the baseline and follow-up. The RI was 2.3±6.5(Table 4).
In the de novo lesions, the proximal reference lumen areas were significantly decreased, whereas the vessel areas were not. In the target lesions, the lumen area decreased and the plaque area increased significantly, whereas the vessel area showed no significant changes (Table 5). The II increased significantly from 15.1±6.3% to 34.9±8.3%, respectively(p=0.001). The RI was -2.5±4.9(Table 5).
A total of 117 patients underwent two or more serial coronary angiograms. The angiographically significant coronary artery disease free survival rate was 99% at 5 year, 89% at 7 year and 71% at 9 year after heart transplantation(Fig. 5).
Transplant coronary artery disease(tCAD) after heart transplantation characteristically displays both diffuse and concentric lesions. Coronary angiogram is not sensitive for detecting tCAD because of the procedure's peculiar nature. Intravascular ultrasound(IVUS) is a more sensitive and accurate tool than coronary angiogram, and especially to detect early tCAD; it can also predict the prognosis in those cases that are without angiographically demon strated disease progression.6) Using IVUS, we found that donor lesion was present in 29%(17/58) and de novo lesion was present in 14%(8/58). The incidence of de novo lesion in our study was much lower than that of western countries. Kapadia reported that de novo lesion occurred in 42% of patients and it was located more in the mid-to-distal segments; further, the de novo lesion was more diffuse and concentric than the donor lesion. More than 60% of the de novo lesions were diffuse and concentric.7) In our series, both the donor lesions and the de novo lesions were not diffuse or concentric, and the target lesions were located mainly at the proximal and proximal-to-mid portions. These findings showed that the incidence and characteristics of CAD after heart transplantation in Koreans were different from those in Western countries.
A recent multicenter IVUS study reported that the progression of intimal thickening over 0.5 mm in the first year after transplantation was a reliable surrogate marker for the subsequent mortality, the nonfatal major adverse cardiac events and the development of angiographic CAD for 5 years after transplantation.8-12) In our study, new development of significant lesion, that is, de novo lesion, occurred in 14% of the patients and donor lesion was present in 29% of the patients. These patients can give us clues about the prognosis of tCAD in Koreans, and this subject requires longer follow-up study.
TCAD is known to show a somewhat different remodeling pattern compared to native coronary artery disease.4)13-16) Coronary arteries in tCAD patients show more negative remodeling, and the remodeling response is inadequate during the 1st year. This remodeling response may contribute to the development of tCAD, and especially during the early period after heart transplantation. For the donor lesions of our study, the vessel area and the intimal thickness were not significantly changed during follow-up, and the remodeling pattern was positive. In the de novo lesions, the plaque area and the intimal index were increased, and the lumen and vessel area was decreased, so the remodeling pattern was negative. When we compared the EP and LP groups, we found that the changes of the plaque burden and the lumen areas were greater in the EP group than in the LP group, whereas vessel area decreased in the EP group and it increased in the LP group. The results showed that at first, in the early phase of heart transplantation, the plaque burden increased, but vessel area did not show a compensatory increase; thus, the remodeling pattern was negative during the 1st year. In the EP group, the remodeling index was negative in 58% of the patients in the EP group. This early phase result was the same as that noted for western countries.4)13-16)
Yet in the later phase after heart transplantation, the remodeling pattern became more positive. The findings at the second year also suggested that rapid progression of intimal thickness occurred mainly during the 1st year of heart transplantation. Our findings were similar to those of a previous report. The different remodeling pattern may also contribute to the development of de novo lesion and the progression of tCAD in Koreans.
When we compared our results with those of Western countries, we observed a lower incidence of de novo lesion and lower proportions of concentric and diffuse lesions. The angiographically significant tCAD-free survival rate in our study was also lower than that in western countries. We don't know the exact reason for these differences, but there are some possible explanations. First, the traditional Korean diet is low in fat and high in vegetables, which is associated with a low incidence of native coronary disease, and this may affect the development of tCAD. Second, the same as for the Chinese people, Koreans have homogenous population and the Korean people's genetic variation is less diverse than that in western populations.17) Third, the incidence of cytomegalovirus(CMV) infection and disease in Koreans is much lower than that in western countries. The pathogenesis of tCAD is multifactorial and CMV infection is one of the possible pathogenetic mechanisms.18)19) Although Koreans usually experience CMV infection before adulthood, and almost all Koreans have anti-CMV IgG antibody in their adult age, the rate of CMV disease in our study was only about 4%. These factors can affect the development of tCAD and also the nature of tCAD in Koreans.
The limitation of this study is that the serial IVUS follow up period was different between groups. So, comparing the IVUS findings from both the early(the first month and one year after) and late(the first one year and over one year after) phases in this study may be not suitable. From this viewpoint, further serial IVUS follow up study and also study on the early and late phase in the same patient are needed.
The incidence and the characteristics of coronary artery disease after heart transplantation in Koreans were somewhat different from those in the western countries. For the post-transplant early period group, the donor lesions developed with the same incidence as in Western countries, but the frequency of de novo lesions was much lower. The incidences of diffuse and concentric lesions were also low; the target lesion sites for both the donor and de novo lesions were mainly located at the mid-to-proximal portions, rather than the distal portions. The progression of transplant coronary artery disease was most severe during the 1st year. The remodeling pattern was negative in the early period, and then it became positive during the late period.
Further study is needed to understand the mechanism and contributing factors for the different nature and incidence of transplant coronary disease between Koreans and Western countries, and longer follow-up study is needed to determine the prognosis of patients with de n ovo lesion or donor lesion.
Figures and Tables
 | Fig. 1Study design. Patients were subgrouped into an EP group and an LP group. Patients in the EP group were analyzed by IVUS within 1 month after transplantation and then 1 year later. Patients in the LP group were analyzed by IVUS 1 and 3 years after transplantation. HTPL: heart transplantation, EP: post-transplant early period, LP: post-transplant late period, IVUS: intravascular ultrasound. |
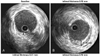 | Fig. 2Intravascular ultrasound (IVUS) images showing the intimal thickness at baseline (A) and after intinal thickness 0.90 mm (B) in one patient, demonstrating de novo lesion. HTPL: heart transplantation, EP: post-transplant early period, LP: post-transplant late period. |
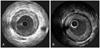 | Fig. 3Intravascular ultrasound (IVUS) showing a coronary arterial eccentric intimal plaque (A) and a concentric intimal plaque (B). |
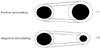 | Fig. 4Calculating the remodeling index (RI). RI was calculated as the change in vessel area/change in the intimal area. An RI ≥1.0 indicates positive remodeling and an RI ≤0 indicates negative remodeling. |
 | Fig. 5Angiographical transplant coronary artery disease (CAD) free survival rate. The angiographically significant coronary artery disease-free survival rate was 99% at 5 year, 89% at 7 year and 71% at 9 year. |
References
1. Bieber CP, Hunt SA, Schwinn DA, et al. Complications in long-term survivors of cardiac transplantation. Transplant Proc. 1981. 13:207–211.
2. St Goar FG, Pinto FJ, Alderman EL, et al. Intracoronary ultrasound in cardiac transplant recipients: in vivo evidence of 'angiographically silent' intimal thickening. Circulation. 1992. 85:979–987.
3. American College of Cardiology. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents developed in collaboratoration with the European Society of Cardiology endorsed by the Society of Cardiac Angiography and Interventions. Eur J Echocardiogr. 2001. 2:299–313.
4. Lim TT, Liang DH, Botas J, Schroeder JS, Oesterle SN, Yeung AC. Role of compensatory enlargement and shrinkage in transplant coronary artery disease. Circulation. 1997. 95:855–859.
5. Hur SH. Lesion characteristics in patients with acute coronary syndrome: a comparison with lesion in patients with stable angina by intravascular ultrasound. Korean Circ J. 2004. 34:548–557.
6. Tuzcu EM, Kapadia SR, Sachar R, et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J Am Coll Cardiol. 2005. 45:1538–1542.
7. Kapadia SR, Nissen SE, Ziada KM, et al. Development of transplantation vasculopathy and progression of donor-transmitted atherosclerosis: comparison by serial intravascular ultrasound imaging. Circulation. 1998. 98:2672–2678.
8. Rickenbacher PR, Pinto FJ, Lewis NP, et al. Prognostic importance of intimal thickness as measured by intracoronary ultrasound after cardiac transplantation. Circulation. 1995. 92:3445–3452.
9. Mehra MR, Ventura HO, Chambers R, et al. Predictive model to assess risk for cardiac allograft vasculopathy: an intravascular ultrasound study. J Am Coll Cardiol. 1995. 26:1537–1544.
10. Wiedermann JG, Wasserman HS, Weinberger JZ. Severe intimal thickening by intravascular ultrasonography predicts early death in cardiac transplant recipients. Circulation. 1994. 90:Suppl I. I93.
11. Anderson TJ, Meredith IT, Uehata A, et al. Functional significance of intimal thickening as detected by intravascular ultrasound early and late after cardiac transplantation. Circulation. 1993. 88:1093–1100.
12. Mainigi SK, Goldberg LR, Sasseen BM, See VY, Wilensky RL. Relative contributions of intimal hyperplasia and vascular remodeling in early cardiac transplant-mediated coronary artery disease. Am J Cardiol. 2003. 91:293–296.
13. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987. 316:1371–1375.
14. Gyongyosi M, Yang P, Hassan A, et al. Arterial remodelling of native human coronary arteries in patients with unstable angina pectoris: a prospective intravascular ultrasound study. Heart. 1999. 82:68–74.
15. Schwarzacher SP, Uren NG, Ward MR, et al. Determinants of coronary remodeling in transplant coronary disease: a simultaneous intravascular ultrasound and Doppler flow study. Circulation. 2000. 101:1384–1389.
16. Schoenhagen P, Ziada KM, Vince DG, Nissen SE, Tuzcu EM. Arterial remodeling and coronary artery disease: the concept of "dilated" versus "obstructive" coronary atherosclerosis. J Am Coll Cardiol. 2001. 38:297–306.
17. Hsu RB, Chu SH, Wang SS, et al. Low incidence of transplant coronary disease in Chinese heart recipients. J Am Coll Cardiol. 1999. 33:1573–1577.
18. Schmid C, Kerber S, Baba HA, Deng M, Hammel D, Scheld HH. Graft vascular disease after heart transplantation. Eur Heart J. 1997. 18:554–559.
19. Park JH, Lee YJ, Kang SJ, et al. Clinical analysis of infections following cardiac transplantation. Korean Circ J. 2001. 31:815–823.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download