Introduction
Idiopathic thrombocytopenia purpura(ITP) is an autoimmune disorder that's characterized by accelerated destruction of platelets. Spontaneous mucocutaneous bleeding is common and death from hemorrhage occurs in approximately 5% of the afflicted individuals.1) Because of the risk of bleeding, aspirin and other pharmacological inhibitors of platelet function are generally contraindicated.
Platelet aggregation plays a crucial role in the pathogenesis of acute and chronic complications following percutaneous coronary intervention(PCI). Moreover, the combination of aspirin and thienopyridine derivatives is essential for minimizing the risk of stent thrombosis(ST).2)
We present here a case of a patient with ITP who underwent PCI and we discuss the management strategies, including antiplatelet therapy.
Case
A 61-year-old woman presented with increasing chest pain and dyspnea upon exertion. The chest pain developed 2 months previously when she was walking uphill or climbing stairs, and this was relieved by several minutes rest. Her pain had been of longer duration and more severe 2 days previous to her hospital presentation. Her chest pain was characterized by a squeezing pattern and this was located in the substernal area and it radiated to her left arm.
On the past history, she was diagnosed with chronic ITP 20 years ago and had taken steroids for this. Despite the maintenance dose of 10 mg prednisolone, there was no increase of the platelet count. She also had diabetes mellitus and hypertension.
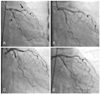
On physical e xamination, she was alert, her blood pressure was 130/80 mmHg with a pulse rate of 66 beats/min. The heart sounds were normal without gallop sounds or significant murmur. The electrocardiography on admission showed no ischemic change. The blood examination showed normal cardiac enzymes: a creatine kinase MB(CK-MB) level of 1.14 ng/mL and a troponin I level of <0.1 ng/mL. The platelet count was 4×109/L. Blood coagulation tests showed a prothrombin time (international normalized ratio) of 0.91 (normal: 0.85-1.13) and an activated partial thromboplastin time of 29.3 seconds(normal: 21-38 seconds). A chest radiograph showed no active lung lesion. Echocardiography revealed normal systolic function without any regional wall motion abnormality and there was a trace of regurgitation at the mitral valve. Diagnostic catheterization was performed through the right femoral artery with using 6 French sheath. Coronary angiography(CAG) revealed 99% stenosis in the middle segment of the left anterior descending artery (LAD) and 95% stenosis in the middle segment of the left circumflex artery(LCX)(Fig. 1A). The right coronary artery showed chronic total occlusion in the posterior descending artery. After the CAG, hematoma developed at the puncture site despite that we had carefully punctured the vessel so as not to injure it and we sealed it with a closure device(Angioseal, St. Jude Medical). PCI was postponed because of the bleeding tendency at the puncture site. We planned to replace the platelets by transfusion and administer intravenous immunoglobulin(IVIG) for preventing bleeding complications, and to deploy bare-metal stents(BMS) in the LAD and LCX lesions. Three days later, the platelet count was elevated to 34×109/L. PCI was performed consecutively for the stenotic lesions of the mid LAD and mid LCX with BMS(Tsunami Gold, Terumo)(Fig. 1B). There was no thrombotic or bleeding complication during or after the procedure. The patient was discharged 3 days later without proscribing any antiplatelet agents. She was instructed to undergo strict hematologic control and we planned to use at least one antiplatelet agent when her platelet count allowed it. But she could not start using any antiplatelet agents during the follow up period because of a persistent, significant low platelet count, which achieved a maximum of 7×109/L. 5 months later, the patient was readmitted due to exertional chest pain she had felt for 4 weeks. On admission, the platelet count was 20×109/L. Follow up CAG showed 95% focal in-stent restenosis in both lesions in which the BMSs had been deployed(Fig. 1C), so we successfully and rather easily performed balloon angioplasty in both lesions(Fig. 1D).
Discussion
Patients with ITP are rarely associated with coronary artery disease.3) If a patient with chronic thrombocytopenia has coronary artery disease, then the concomitance of known coronary risk factors, such as a family history for cardiovascular disease, hypertension, diabetes, dyslipidemia or cigarette smoking, should be considered.4) Fruchter et al.5) reported that antigenic mimicry between platelets and endothelial cells plays a role of the pathogenesis of AMI in thrombocytopenic patients suffering with chronic ITP.5)
One clinical problem of ITP is the bleeding tendency. Therefore, appropriate treatments are needed to increase the platelet counts at the time of interventional therapy. Some such cases have safely undergone coronary artery bypass grafting(CABG) with prophylactic treatment(steroid, immunosuppressants, immunoglobulin therapy or platelet transfusion) to increase the platelet count to greater than 50×109/L so the patient can achieve hemostasis.6)7)
Performance of PCI in a patient with ITP is another unique situation in which the platelet function needs to be sufficiently inhibited to prevent stent thrombosis(ST), but not to the extent of causing bleeding complications. Our patient was refractory to steroid therapy and the platelet count was only 4×109/L despite of long-term maintenance of her steroid therapy; this very well could provoke spontaneous bleeding, for example, intracranial hemorrhage, acute gastrointestinal bleeding and/or bleeding at the arterial access site. Actually, hematoma developed at the puncture site despite performing careful puncturing and sealing with a closing device(Angioseal, St. Jude Medical). We decided not to administer any antiplatelet agents, including aspirin and clopidogrel, because of the risk of bleeding complications; we replaced the platelets by transfusion and administered IVIG for 3 days. Because IVIG therapy for ITP has been associated with a rare occurrence of thrombotic events such as myocardial infarction and stroke,8)9) careful attention must be paid to a rapid rise of platelet count, particularly in the elderly and in those patients with coronary risk factors.
There may be several therapeutic options for patients with ITP and who undergo PCI, such as percutaneous transluminal coronary balloon angioplasty(PTCA) or stent implantation(BMS or DES). We chose BMS implantation in both stenotic lesions because of its lower rate of restenosis compared to the PTCA.10) Although DES shows a lower restenosis rate and a similar ST rate in the first 30 days after stent implantation compared with BMS,11) the DES needs long-term maintenance with antiplatelet agents to prevent ST and if this is not done, the incidence of ST is markedly increased after discontinuation of antiplatelet agents.12)13) Iakovou et al.14) reported that ST occurred in 29% of the patients treated with DES and who prematurely discontinued dual antiplatelet therapy; the clinical consequences were severe indeed, with a case-fatality rate of 45%.
Although focal in-stent restenosis developed 5 months later in both lesions in which the BMSs were deployed, there was no episode of ST despite of not using any antiplatelet agents during the follow up period. We successfully performed PTCA in both lesions quite easily.
In the best of our knowledge, this is the first report of a patient with severe, refractory, chronic ITP who was treated with BMS implantation and no antiplatelet agents, and there was no epidose of ST.
In the literature on patients with ITP who underwent PCI, Caputo et al.15) reported the case of a 62-year-old male with refractory ITP together with unstable angina. Despite a low platelet count of 3×109/ L, coronary stenting was successfully performed in the LAD and the patient tolerated 4 weeks of aspirin and 2 weeks of clopidogrel without bleeding complications. Kikuchi et al.16) reported the case of a 68-year-old female with a platelet count of 22×109/L who underwent stent deployment for the treatment of an LAD occlusion. She was treated with ticlodipine without any bleeding complications. The authors of above studies thought that the ST risk overweighed the risk of life threatening bleeding over a short duration of treatment with antiplatelet agents. However, bleeding complications can lead to serious clinical consequences and the ST rate in patients with thrombocytopenia might be lower than that of patients with normal platelet counts. Therefore, we thought that antiplatelet agents should be considered on a case by case basis according to the patient's condition, for example, the platelet count and bleeding tendency.
Marques et al.17) reported the case of a 54-year-old male with a platelet count of 8×109/L, and he underwent BMS deployment. Although the patient had a significantly low platelet count, they tried to using at least enoxaparin because 2 BMSs were implanted in the LAD and LCX. However, the occurrence of epistaxis caused suspension of that drug, and the patient was maintained without antiplatelet agents during the follow up period of 4 months. Stouffer et al.18) reported the case of a 77-year-old male with a platelet count of 70×109/L who underwent only cutting balloon angioplasty and he was on chronic aspirin therapy(81 mg q.d). 5 weeks later, the patient received a BMS(MultiLink Penta, Guidant) in the setting of restenosis. Following the procedure, the patient was treated with aspirin plus clopidogrel. Three weeks later, the patient developed diffuse petechiae and a spontaneous epistaxis. The clopidogrel was discontinued and aspirin was withheld for 4 days; since that time, he had no further bleeding problems and he continued on aspirin. We tried using antiplatelet agents after stent implantation for fear of ST, but bleeding complications developed. In this case, we considered it prudent not to use antiplatelet agents because of the low platelet count and the bleeding tendency at the puncture site. In summary, we report here on a case of a patient with ITP who underwent PCI with using BMS. Although focal instent restenosis developed 5 months later in both lesions in which the BMSs were deployed, there was no episode of ST despite not using any antiplatelet agents during the follow up period. The present case suggests that the rate of stent thrombosis may be lower in patients with a low platelet count than that in patients with a normal platelet count, and antiplatelet therapy may be considered on a case by case basis for patients with thrombocytopenia. Furthermore, PCI with using BMS was an attractive therapeutic option despite the risk of ST when managing patients with chronic, refractory ITP and who have a significantly low platelet count and a high bleeding tendency, but not enough to prohibit using antiplatelet agents.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download