Introduction
Esophageal hematoma, a rare clinical entity, is thought to be generally the result of esophageal injury.1) The majority of cases of esophageal hematoma have been associated with certain predisposing factors. The most common predisposing factors are esophageal instrumentation,2) hematologic or bleeding disorders,3) and anticoagulation therapy.4) Endoscopy and chest computed tomography (CT) are normally necessary for the establishment of a diagnosis. In this report, we describe a patient with esophageal hematoma that was discovered by a bedside transthoracic echocardiography (TTE). TTE provides detailed anatomic and functional information regarding the cardiac and pericardial5) structures. TTE proved helpful in the early detection of esophageal hematoma and differential diagnosis from other pathologies by demonstrating the complete resolution of the mass on a follow-up study.
Case
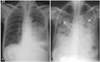
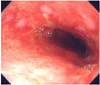
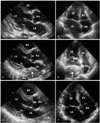
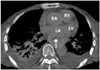
A 72-year-old woman presented with chronic generalized weakness and poor oral intake. The patient had a history of gastric cancer which was cured by gastrectomy 20 years ago, and also a history of iron-deficiency anemia. On hospital day 2, the patient developed heartburn and dyspnea. The physical examination revealed fever (38℃), tachypnea (24 breaths/min), tachycardia (105 beats/min), and shock (80/50 mmHg). Chest radiography evidenced pneumonic consolidation at the left lower lung field (Fig. 1A). A diagnosis of aspiration pneumonia with septic shock was made on the basis of the clinical and radiological findings. The patient was treated with intravenous (IV) antibiotics and inotropic support. However, on hospital day 6, the patient developed severe dyspnea with generalized edema. Chest radiography revealed diffuse bilateral pulmonary edema (Fig. 1B). She was intubated and maintained with mechanical ventilation. A bedside TTE revealed akinesia of the mid and apical portions of the left ventricle (LV). The ejection fraction (EF) was 25%. The patient had no previous history of congestive heart failure or coronary artery disease, and the cardiac enzyme results were also negative. Therefore, she received conservative treatment, including judicious volume control and inotropic support for possible stress-induced cardiomyopathy. The patient could be extubated after 6 days (on hospital day 12), and a follow-up TTE evidenced nearly normalized LV function with an EF of 52%. The patient was transferred to the general ward without inotropic support, but developed severe aspiration pneumonia 2 days later (on hospital day 14) and was again intubated. A bedside TTE conducted after the second intubation revealed normal LV function without any regional wall motion abnormalities (Fig. 3A, B). Broad spectrum antibiotic treatment was initiated for nosocomial pneumonia, and a feeding tube was inserted for enteral feeding. Unfortunately, the patient's clinical condition deteriorated slowly, and thus she could not be weaned from mechanical ventilation. After approximately 1 month (on hospital day 40), the patient developed severe shock, and some fresh blood was observed in the feeding tube. She had been on IV heparinization due to prophylaxis of deep vein thrombosis (DVT) for two weeks. Heparin treatment was discontinued and an endoscopy was conducted in order to rule out gastrointestinal bleeding. No active lesions were observed with the exception of mild esophagitis, which was attributed to injury caused by the feeding tube (Fig. 2). We noted no bleeding events thereafter, and enteral tube feeding was maintained. After 5 days (on hospital day 45), a follow-up bedside TTE was conducted for sustained shock and the possible recurrence of stress-induced cardiomyopathy. This demonstrated preserved cardiac function, but an unexpected mass approximately 5.4×3.3 cm in size compressing the left atrium (LA) was discovered (Fig. 3C, D). A chest CT scan was conducted in order to evaluate the possibility of esophageal hematoma or cancer (Fig. 4).
An experienced radiologist suggested that the mass was more likely to be a hematoma, and we concluded that the mass was indeed an esophageal hematoma because of the prolonged tube feeding and IV heparinization. The feeding tube was removed and IV heparinization was discontinued. Nutritional support was supplied only via parenteral feeding. IV hydration and inotropic support was continued for the treatment of sustained shock. After 1 week (on hospital day 52), a follow-up TTE revealed that the mass had been resolved completely (Fig. 3E, F), and therefore confirmed that the mass was an esophageal hematoma. However, inotropic support could not be discontinued due to the severe septic shock. Despite appropriate treatment for pneumonia, the patient's condition worsened. Unfortunately, the patient died as the consequence of severe nosocomial pneumonia after 1 month (on hospital day 84) of prolonged treatment.
Discussion
Esophageal hematoma, a rare clinical entity, is thought to be generally attributable to esophageal injuries.1) The first case of esophageal hematoma was reported in 1957.6) As of 2000, 174 cases were reported worldwide.7) Esophageal hematoma typically affects middle-aged or elderly women, but the majority of patients with this condition are in the seventh or eighth decades of life.8) The majority of cases of esophageal hematoma have predisposing factors. The most common predisposing factors are esophageal instrumentation,2) hematological or bleeding disorders,3) and anticoagulation therapy.4) Other less frequent causes include trauma,9) foreign body ingestion,10) and vomiting.11) In this case, the esophageal hematoma was developed in a 72 year-old female patient with prolonged enteral tube feeding and IV heparinization for the prophylaxis of DVT in critically ill condition.
The symptoms of esophageal hematoma include retrosternal chest pain, dysphagia, and hematemesis. 35% of patients present with this triad, and half present with at least two symptoms. Incidental cases without symptoms, such as the case with our patient, were also reported.12)
The prognosis of esophageal hematoma is not particularly bad. Conservative care remains the principal therapeutic modality. The majority of hematomas resolve spontaneously, and less than 15% of patients will ultimately require surgery.11)
As patients with esophageal hematoma often present with retrosternal chest pain, the diagnosis of esophageal hematoma is usually delayed until other life-threatening conditions have been excluded. This condition can be confused with myocardial infarction (MI), aortic dissection, dissecting aortic aneurysm, aortoesophageal fistula, pulmonary embolism, esophageal perforation, or esophageal cancer.4)11) The recognition and accurate diagnosis of esophageal hematoma is critical, as conservative treatment is the mainstay of management. Further invasive investigations or treatments can be potentially dangerous.13)
Endoscopy is a standard modality for the diagnosis of esophageal hematoma. These hematomas appear round or longitudinal in shape, with a blue-colored submucosal elevation, with or without a tear.1) However, endoscopy is often delayed or contraindicated when MI, aortic dissection/aneurysm, or esophageal perforation are suspected and cannot be easily conducted in critically-ill patients, as in this case. Furthermore, the appearance at endoscopy can be mistaken for an esophageal malignancy or aortoesophageal fistula from an aortic aneurysm.14) Although endoscopy was conducted 5 days prior to TTE and the hematoma may not have been developed at that time, endoscopy may have missed the hematoma. Therefore, we utilized TTE as a follow-up study and TTE was easier to repeatedly conduct than endoscopy in our critically-ill patient.
Chest CT is useful for the evaluation of aortic and mediastinal pathologies. Esophageal hematoma appears as an eccentric, well-defined, intramural mass which extends along a considerable length of the esophagus. The mass has the density of either blood or water. It is of typical attenuation prior to contrast enhancement, and there is no enhancement after contrast administration.15) However, the findings of the CT may be misinterpreted by less experienced examiners as a neoplasm. An experienced radiologist in our case suggested that the mass was more likely to be a hematoma, in addition to the fact that our patient had been on prolonged tube feeding and IV heparinization. Therefore, we concluded that the mass was indeed an esophageal hematoma.
TTE is not a routine modality for the diagnosis of esophageal hematoma. However, it provides high-resolution images of cardiac and pericardial5) structures, such that detailed anatomic and functional information regarding the heart, pericardium, aorta, and esophagus can be obtained. It is particularly useful in the differential diagnosis of MI, aortic dissection, aortic aneurysm, and pulmonary embolism.16) This can be conducted even in hemodynamically unstable patients. TTE detected the esophageal mass first, and was easier to repeatedly conduct than was endoscopy or CT, without renal toxicity or radiation hazards in our critically-ill patient. It also demonstrated the complete resolution of the mass, therefore confirming that the mass was an esophageal hematoma.
In this case, TTE proved helpful in the early detection, differential diagnosis, and follow-up of esophageal hematoma.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download