Abstract
Abciximab is one of the glycoprotein IIb/IIIa receptor inhibitors, and it is known to be effective for preventing and treating the thrombotic complications of percutaneous coronary intervention (PCI). On the other hand, there is an increasing risk of hemorrhagic complications when using abciximab, especially in the case of abciximab-induced thrombocytopenia. Acute profound thrombocytopenia is a rare, but life threatening adverse reaction to abciximab, and it can even occur within a few hours of the first exposure. We report here on a case of 56 year-old woman who experienced massive bleeding of her brachial artery access site. This was caused by abciximab-induced acute profound thrombocytopenia after performing PCI concomitant with using abciximab.
Abciximab is a widely used glycoprotein IIb/IIIa receptor inhibitor, and it is the Fab fragment of the chimeric monoclonal antibody 7E3. It inhibits platelet aggregation and activation.1) It has been used to prevent and treat peri-procedural thrombotic complications in the setting of acute coronary syndrome and percutaneous coronary intervention (PCI).2) As the clinical usage of abciximab has expanded, there is an increased risk of varying degrees of hemorrhagic complications because of its potent antiplatelet effect. In addition, the acute onset of profound thrombocytopenia, which is a rare adverse reaction to abciximab, can cause fatal hemorrhagic complications.3) We experienced a case of serious hemorrhagic complication of the vascular access site that resulted in compartment syndrome of the forearm. It was caused by abciximab-induced acute profound thrombocytopenia when we used abciximab to treat acute stent thrombosis. We report here on the treatment and clinical course of this rare adverse reaction to abciximab, along with a review of the relevant literature.
A 56-year-old female visited our hospital due to chest pain upon exertion. She had been taking several anti-hypertensive medicines, but she had no history of diabetes or smoking. On admission, her blood pressure, heart rate and respiratory rate were 130/90 mmHg, 80 beats/minute and 16 times/minute, respectively. A physical examination showed no abnormality. A complete blood cell (CBC) count showed the hemoglobin level was 13.0 g/dL, the platelet count was 230,000/mm3 and the white blood cell count was 7,040/mm3. The cardiac enzymes and blood chemistry were within normal limits. The prothrombin time (PT) and activated partial thromboplastin time (aPTT) also were within normal limits. The baseline electrocardiogram (ECG) was normal (Fig. 1). A MIBI-SPECT myocardial perfusion scan showed a reversible perfusion defect of the anterior wall of the left ventricle.
The patient had received aspirin (200 mg) and clopidogrel (300 mg) the day before undergoing a coronary angiogram. A bolus of 3,000 U of heparin was administered and systemic heparinization was performed using a continuous infusion of 12 U/kg/Hr, and this was titrated to achieve a partial thromboplastin time that was twice the control value.
A coronary angiogram (CAG) was performed. At first, we tried a radial approach, but we failed to access the radial artery due to vasospasm and intimal dissection. We then accessed the brachial artery. A baseline CAG showed about 85% segmental stenosis of the middle left anterior descending artery (LAD) (Fig. 2A). PCI was then performed. A 5 French Judkins left 4 guiding catheter (Johnson and Johnson, USA) was engaged into the left coronary artery and a guidewire (Whisper®, Guidant, USA) was inserted across the lesion. After balloon dilatation, a 3.0×18 mm sized Cypher® stent (Johnson & Johnson, USA) was implanted at 16 atmospheres (atm). The final CAG showed no significant residual stenosis and no intimal dissection of the coronary artery (Fig. 2B). She was transferred to the intensive care unit and we removed the vascular sheath from the brachial artery and applied a sandbag after performing manual compression for over 30 minutes.
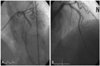
She then complained of severe chest pain and the ECG showed a significant ST elevation from the V1 to the V5 leads (Fig. 3). Acute thrombotic closure of the stent was strongly suspected, and so she was re-transferred to the cardiac catheterization laboratory. We accessed a femoral artery at that time. A CAG showed thrombosis and subtotal occlusion (over 90%) of the distal portion of the stent (Fig. 4A). An intravenous abciximab infusion was promptly initiated and thrombosuction was performed using a suction catheter. After thrombosuction, TIMI 2 flow of the LAD and the diagonal branch were restored, but spiral dissection was noted that originated from the distal edge of the stent to the distal diagonal branch. We decided to implant an additional stent in the dissected portion of the LAD. A 2.75×33 mm sized Cypher® stent (Johnson & Johnson, USA) was implanted in the LAD at 12 atm. After additional stenting, the blood flow in both the LAD and the diagonal branch were restored to TIMI 3 flow without ant residual stenosis or dissection (Fig. 4B).
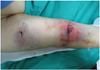
The procedure was finished and an ECG showed normalized ST segments. Her chest pain subsided, but severe bleeding developed 30 minutes later in the brachial arterial access site. Despite performing manual compression for over 2 hours, the bleeding was not controlled (Fig. 5). She complained of severe pain in her arm and sensory changes in her 1st, 2nd and 3rd fingers. A CBC showed an acute onset of severe thrombocytopenia (5,103/mm3). The abciximab was stopped immediately and 8 units of concentrated platelets were transfused. Four hours after transfusion, her bleeding stopped and the platelet counts increased up to 115,000/mm3. We evaluated other possible causes of her thrombocytopenia. To rule out ethylene diamine tetraacetic acid (EDTA)-dependent in vitro platelet clumping, we repeatedly checked the peripheral blood smears and platelet counts with citrate (Fig. 6). The markers of the disseminated intravascular coagulation were within normal limits and any anti-platelet antibodies were not detected. We considered performing surgical decompression if the development of elevated intra-muscular pressure of the right arm threatened to cause serious neurological and circulatory damage to the forearm. We kept the patient under observation and there was no progression towards further neurological damage. A week later, the sensory changes in her fingers recovered and her platelet counts were normalized without any additional transfusions (Fig. 7). She was discharged without any other peri-procedural event and she has been followed up at the outpatient clinic.
There are several kinds of glycoprotein IIb/IIIa receptor blocking agents such as abciximab, tirofiban and eptifibatide. Several clinical trials have shown them to be effective for inhibiting platelets.4) These agents inhibit platelet aggregation by preventing the binding of the Von Willebrand factor, fibrinogen and other adhesive molecules to the glycoprotein IIb/IIIa receptor of activated platelets. They also block the generation of thrombin that follows platelet activation.1)10) Hemorrhagic complications occasionally result in serious clinical events such as intracranial hemorrhage, pulmonary hemorrhage, gastrointestinal hemorrhage and massive hematoma formation at the vascular access site. Major bleeding is defined as intracranial hemorrhage or a decrease of over 5 g/dL in the hemoglobin level or a decrease of over 15% in the hematocrit, and this condition requires a transfusion. Major bleeding complications are reported in about 3.5% of these patients.5) In addition, acute onset thrombocytopenia, which is a rare adverse reaction to abciximab, can cause fatal hemorrhagic complications; moreover, an adverse reaction is unpredictable. Appropriate treatment and careful monitoring should be initiated as soon as possible. The risk factors for thrombocytopenia when using abciximab are known to be an age >65 years old, a weight <90 kg and a baseline platelet count <200,000/mm3.3)6) The specific mechanisms of abciximab-induced thrombocytopenia are unknown.
Because of the concomitant use of heparin and abciximab in this case, we had to distinguish between heparin-induced thrombocytopenia (HIT) and abciximab-induced thrombocytopenia (AIT). Two types of HIT have been described. Type I is characterized by a mild transient thrombocytopenia. Type II is caused by an IgG-mediated immune reaction, and this is a disorder with a delayed onset, but it is persistent. Type II HIT is often associated with acute thromboembolic events. There is no test that can distinguish HIT from AIT when both heparin and abciximab are given. However, AIT behaves differently from HIT. AIT occurs rapidly after the administration of abciximab, while HIT develops over a period of days, HIT is not acute and it may be associated with bleeding and thrombosis. Knowledge about the patient's exposure to heparin within the last 2 months is also helpful to make this discrimination.7) Pseudothrombocytopenia is another important disease in the differential diagnosis of AIT. It is caused by EDTA-dependent in vitro platelet clumping and it can be detected by a peripheral blood smear and repeatedly checking the platelet count by using blood that is anticoagulated with citrate.8) The prothrombin time, the fibrinogen level, the D-dimer and the fibrin degradation product (FDP) should be checked in order to rule out disseminated intravascular coagulation.9)
The management of profound thrombocytopenia is controversial. Platelet transfusion and immediate cessation of treatment appear to be the most effective measures. It is currently unclear if other antiplatelet therapies should also be suspended. Intravenous IgG can be considered in addition to platelet transfusion, but the therapeutic effect of the IgG is unknown.
Figures and Tables
Fig. 2
AP cranial view. A CAG of the left coronary artery. A: the baseline CAG revealed about 85% stenosis of the middle LAD. B: after stent implantation, a CAG revealed no residual stenosis of the LAD. AP: anteroposterior, CAG: coronary angiogram, LAD: left anterior descending.
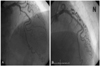
Fig. 3
When chest pain developed after PCI, an electrocardiogram showed ST segment elevations in the anterior chest leads. PCI: percutaneous coronary intervention.

Fig. 4
AP cranial view. A CAG of the left coronary artery. A: an emergency CAG showed subtotal occlusion (>90%) of the middle LAD, which was previously a stent implantation site. B: after the abciximab infusion, thrombosuction and another stent implantation, the CAG showed no residual stenosis or dissection with restored TIMI 3 flow. AP: anteroposterior, CAG: coronary angiogram, LAD: left anterior descending, TIMI: thrombolysis in myocardial infarction.
References
1. Coller BS. Blockade of platelet GPIIb/IIIa receptors as an antithrombotic strategy. Circulation. 1995. 92:2373–2380.
2. The EPIC investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994. 330:956–961.
3. Makoni SN. Acute profound thrombocytopenia following angioplasty: the dilemma in the management and review of the literature. Heart. 2001. 86:E18.
4. Topol EJ, Byzova TV, Plow EF. Platelet GPIIb-IIIa blockers. Lancet. 1999. 353:227–231.
5. Fefguson JJ, Kereiakes DJ, Adgey AA, et al. Safe use of platelet GP IIb/IIIA inhibitors. Eur Heart J. 1998. 19:D40–D51.
6. Keriakes DJ, Berkowitz SD, Lincoff AM, et al. Clinical correlates and course of thrombocytopenia during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade. Am Heart J. 2000. 140:74–80.
7. Claeys LG, Berg W. Major bleeding and severe thrombocytopenia after combined heparin and abciximab-c7E3 Fab therapy. Eur J Vasc Endovasc Surg. 2003. 25:85–87.
8. Christopoulos CG, Machin SJ. A new type of pseudothrombocytopenia: EDTA-mediated agglutination of platelets bearing Fab fragments of a chimeric antibody. Br J Haematol. 1994. 87:650–652.
9. Jubelirer SJ, Koenig BA, Bates MC. Acute profound thrombocytopenia following C7E3 Fab (abciximab) therapy: case reports, review of the literature and implication for therapy. Am J Hematol. 1999. 61:205–208.
10. Kim W, Jeong MH, Kim KH, et al. The rescue use of a patient glycoprotein IIb/IIIa receptor blocker in high risk patient with acute myocardial infarction underwent percutaneous coronary intervention. Korean Circ J. 2001. 31:492–499.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download