Abstract
We report a case of hypertensive heart failure with severe stenosis of the descending aorta. The patient had hypertension; however, he had not previously received any antihypertensive treatment. After receiving antihypertensive therapy for 2 weeks, he was admitted to our hospital for acute heart failure. Computed tomography (CT) and magnetic resonance imaging (MRI) showed severe arteriosclerotic stenosis of the descending aorta above the renal artery bifurcation. He underwent aortic resection and grafting. After surgery, his condition improved, and he was discharged.
When patients are admitted to an emergency room for dyspnea, various conditions have to be differentiated. If it is due to cardiac causes, it has to be assessed whether the causative condition is acute myocardial infarction, valvular heart disease, or exacerbation of an already existing cardiac disease. Coronary artery diseases are the most common cause of heart failure; however, valvular heart disease, hypertension, congenital heart disease, drugs, infection, and metabolic disease should be considered as the differential diagnoses.1) We encountered the case of a 66-year-old male patient who had hypertensive heart failure with severe arteriosclerotic stenosis of the descending aorta. No such cases have been reported in Korea. Therefore, we report here, the case together with a review of the literature.
A 66-year-old male patient visited our emergency room for sudden onset of dyspnea. On inquiry into the past history, he revealed that he had diabetes and hypertension but had not received any treatment for them. He was a heavy smoker and had a history of smoking for 60 pack-years. He had visited the outpatient clinic of our hospital 2 weeks ago for mild exertional dyspnea. At that time, his heart beat was normal; however, a grade 2/6 pansystolic murmur was heard on the left side of the chest. The lung fields were clear. In the outpatient clinic, the blood pressure measured in the left arm was 160/100 mmHg. On electrocardiography (ECG), except left ventricular hypertrophy by voltage criteria, no special findings were detected. Echocardiography also revealed no specific findings except left ventricular hypertrophy. In the outpatient clinic of our hospital, calcium channel blocker and diuretic were prescribed for the control of hypertension.
At the time of his visit to the emergency room, physical examination revealed a high blood pressure of 240/120 mm Hg in the left upper limb. Rales were heard in both lung fields, and a grade 4/6 pansystolic murmur was auscultated on the left side of the chest. Chest X-ray showed cardiomegaly and pulmonary congestion (Fig. 1A). The ECG was not different from the one obtained previously in the outpatient clinic. On emergency echocardiography, the valve function was normal, and no regional wall motion abnormalities were detected; however, diffuse hypokinesia was revealed, and the left ventricular ejection fraction was 40%; this suggested deterioration in comparison to the left ventricular ejection fraction measured by echocardiography 2 weeks before, i.e., 58%. On laboratory studies, serum creatinine (Cr) was 1.3 mg/dL. Troponin-I and creatine kinase (CK)-MB were within the normal range. Serum B-type natriuretic peptide (BNP) was elevated to 1246 pg/mL. Arterial blood gas analysis on room air yielded a pH of 7.313, pCO2 of 39.9 mmHg, pO2 of 41.9 mmHg, bicarbonate level of 20.0 mmol/L, and 73.5% oxygen saturation; moreover, since acute dyspnea was the symptom, endotracheal intubation was performed and ventilatory support was provided.
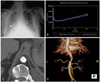
During his hospital stay, angiotensin receptor blockers (ARB) were administered to lower the blood pressure, and on the next day, serum Cr increased from 1.3 mg/dL to 2.2 mg/dL. Therefore, we suspected secondary hypertension due to renal artery stenosis, and a renal Doppler ultrasound was performed; however, renal artery stenosis was absent. Captopril renal scan showed positive findings (Fig. 1B). Aortic computed tomography (CT) showed calcified arteriosclerosis in the suprarenal abdominal aorta along with severe narrowing (Fig. 1C). Aortic magnetic resonance imaging (MRI) revealed no stenosis of the renal arteries and a 90% stenosis of the part of the descending aorta immediately above the renal arteries (Fig. 1D). Plasma renin activity was 11.4 ng/mL/h (normal value, 0.68-1.36 ng/mL/h), and serum aldosterone was 210.6 pg/mL (normal value, 35.7-240 pg/mL).
Based on the above findings, hypertensive heart failure with severe stenosis of the descending aorta was diagnosed. Therefore, we performed thoracoabdominal aorta replacement surgery using an artificial graft (Fig. 2A). Aortic CT performed 20 days after surgery showed no aortic stenosis (Fig. 2B). Histopathological examination of the resected specimen showed severe arteriosclerosis.
After surgery, there was no dyspnea and no systolic murmur on auscultation. The blood pressure and serum Cr returned to normal without any drug treatment, and cardiomegaly was shown to have improved on chest X-ray. Echocardiography conducted 1 month after surgery showed no abnormality with normal wall motion and left ventricular ejection fraction, and serum BNP was 82 pg/mL. Currently, he is in a good state and is under follow-up.
In Korea, 2 cases of arteriosclerotic stenosis of the descending aorta have been reported. In these cases, the clinical pattern followed a chronic course manifesting as claudication of the lower extremity or hypertension.2) However, in our case, the clinical condition of the patient followed an acute course, i.e., acute heart failure. This was particularly different from the previously reported cases, and no clinical symptoms or signs were caused by the stenosis of the descending aorta except hypertension.
Impaired left ventricular function has been reported to induce chronic heart failure in patients with stenosis of the descending aorta.3) However, in our patient, acute heart failure developed within 2 weeks. It is thought that blood pressure needs to be maintained for ensuring sufficient blood flow to the lower part of the abdominal aorta through the stenotic segment. However, after antihypertensive treatment, the blood flow to the abdominal aorta decreased, resulting in reduced renal perfusion; this induced functional renal artery stenosis and activated the renin-angiotensin-aldosterone system, leading to rapid development of acute heart failure.
The diseases showing similar symptoms are middle aortic syndrome (MAS) and midaortic dysplastic syndrome, which are clinical conditions caused by the narrowing of the descending thoracic or abdominal aorta. Major arterial branches and visceral arteries (renal, superior mesenteric, or hepatic) may be involved.4) The etiology of this syndrome is controversial. Some authors report a congenital origin associated with conditions such as neurofibromatosis and Williams syndrome and acquired causes such as arteritis (Takayasu's arteritis and temporal arteritis) or fibromuscular dysplasia.5) Patients present with systemic hypertension, claudication of the lower limb, and headache, and a systolic murmur arising from the stenotic area can be auscultated. Aortic narrowing can be shown and diagnosed on CT, MRI, and aortography.6) Symptomatic treatment consists of antihypertensive medication, and the causal treatment is either percutaneous transluminal angioplasty or surgery comprising reconstruction of the aorta along with the resection of the stenotic segment.7-9) It is not clear whether our case should be included under MAS. Thus far, the major causes of MAS have been reported to be coarctation of the aorta due to congenital diseases9-11) or stenosis due to arteritis such as Takayasu's arteritis.12-14) Presently, it is controversial whether the stenosis of the descending aorta caused by arteriosclerosis can indeed be included under MAS. Cases of MAS over 40 years of age at presentation have rarely been reported. However, considering that the clinical symptoms of our patient developed due to stenosis of the descending aorta, it may be considered to be MAS in a broad sense.
In cases where serum Cr increases rapidly after the administration of angiotensin-converting enzyme inhibitors (ACEi) or ARB in a hypertensive patient, we should thoroughly investigate the patient to rule out functional or anatomical renovascular hypertension. In such cases, the use of antihypertensive agents can induce acute renal failure, heart failure, and ischemia in the lower extremity; hence, special precautions should be taken to prevent the side effects of these drugs.
In our case, although a pansystolic murmur was detected at the time of the visit to the emergency room, the lesion that may have caused the murmur was not detected on echocardiography, and treatment was delayed. When hypertensive patients are admitted for acute heart failure, if a murmur is heard on physical examination, and the lesion that may have caused the murmur cannot be detected on routine investigations, we should suspect acute heart failure related to aortic stenosis. Measurement of blood pressure in both upper and lower extremities is very important, and sometimes critical, in correctly diagnosing hypertensive heart failure.
Figures and Tables
Fig. 1
Chest X-ray on admission showing pulmonary edema and cardiomegaly (A). Captopril renal scan. After captopril injection, an increase in DTPA accumulation was noted (B). Aortic CT (C) and aortic MRI (D) showing arteriosclerosis with marked stenosis in the thoracoabdominal part of the aorta. DTPA: diethylene triamine pentaacetic acid, CT: computed tomography, MRI: magnetic resonance imaging.
References
1. Onwuanyi A, Taylor M. Acute decompensated heart failure: pathophysiology and treatment. Am J Cardiol. 2007. 99:25D–30D.
2. Kim S, Ha JW. Middle aortic syndrome: report of 4 cases. Korean Vascular Sur. 1995. 11:230–237.
3. Seki A, Ogawa H, Fujiu K, Kawagoe Y, Kasanuki H. Middle aortic syndrome diagnosed at 54 years of age. Angiology. 2002. 53:605–608.
4. Kumar S, Bury RW, Roberts DH. Unusual case of refractory hypertension: late presentation of the mid-aortic syndrome. Heart. 2002. 87:E3.
5. Connolly JE, Wilson SE, Lawrence PL, Fujitani RM. Middle aortic syndrome: distal thoracic and abdominal coarctation, a disorder with multiple etiologies. J AM Coll Surg. 2002. 194:774–781.
6. Lewis VD, Meranze SG, McLean GK, O'Neill JA, Berkowitz HD, Burke DR. The midaortic syndrome: diagnosis and treatment. Radiology. 1988. 167:111–113.
7. Cho YK, Lee YG, Song KS, et al. Balloon dilatation angioplasty of aortic coarctation in adult. Korean Circ J. 1994. 24:681–686.
8. Delis KT, Gloviczki P. Middle aortic syndrome: from presentation to contemporary open surgical and endovascular treatment. Perspect Vasc Surg Endovasc Ther. 2005. 17:187–203.
9. Lim JH, Jeong MH, Park WS, et al. A successful stening of the coarctation of the distal thoracic aorta in an adult. Korean Circ J. 2004. 34:420–424.
10. Glenn F, Keeper EB, Speer DS, Dotter CT. Coarctation of the lower thoracic aorta immediately proximal to celiac axis. Surg Gynecol Obstet. 1952. 94:561–569.
11. Poulias GE, Polemis L, Skoutas B, Doundoukia N, Papayoannou K. Coarctation of the aorta of unusual morphology. J Cardiovasc Surg. 1984. 25:211–215.
12. Kim DK, Kim YJ, Ryu JS, Eom WS, Cho JH, Jeong YT. Middle aortic syndrome diagnosed at 51 years of age. Korean J Med. 2004. 66:293–297.
13. Messina LM, Reilly LM, Goldstone J, Ehrenfeld WK, Ferrell LD, Stoney RJ. Middle aortic syndrome: effectiveness and durability of complex arterial revascularization technique. Ann Surg. 1986. 204:331–339.
14. Lande A. Takayasu's arteritis and congenital coarctation of the descending thoracic and abdominal aorta: a critical review. AJR Am J Roentgenol. 1976. 127:227–233.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download