Abstract
Background and Objectives
Intravascular ultrasound (IVUS) can be useful for assessing the causes of in-stent restenosis (ISR) after sirolimus-eluting stent (SES) implantation. We used IVUS to evaluate the causes of ISR after SES implantation.
Subjects and Methods
SES implantation was performed in 502 patients with 670 coronary lesions. Of these patients, 27 patients had angiographic ISR in 28 lesions. We evaluated the patterns of ISR and we wanted to elucidate the possible mechanism of ISR after SES implantation with using IVUS analysis.
Results
The ISR pattern was focal in 26 lesions, and diffuse in 2 lesions, including 1 total occlusion. When analyzing the 21 IVUS-applicable lesions, stent underexpansion [the minimal stent cross-sectional area (CSA) was <5 mm2 and it was <4.5 mm2 in the cases of small coronary arteries (reference vessel diameter <2.8 mm)] was observed in 10 lesions (48%). Stent fracture (defined as non-visualization of the struts on IVUS at the restenotic segments) and edge restenosis was identified in the 3 (14%) and 3 lesions (14%), respectively. Except for edge stenosis, stent underexpansion was observed in 55% of the intra-stent restenotic lesions and it was more prominent in the small coronary arteries (7/8 small coronary artery lesions). Stent underexpansion, stent fracture or edge restenosis were not related to the 7 ISR lesions (33%) in which profound intimal hyperplasia within the stent occurred.
Several studies have demonstrated that Sirolimus-eluting stents(SES, Cypher, Cordis/Johnson and Johnson, Miami, FL) strongly suppress neointimal hyperplasia and effectively reduce in-stent restenosis(ISR).1-4) However, ISR after SES implantation does occur in some cases and restenosis is still a major problem in the era of drug-eluting stent(DES).5)6) Although attempts have been made to find the causes of ISR after SES implantation and some risk factors have been suggested as the predictors of ISR,4)6-8) the predictors of ISR in de novo lesion after SES implantation, as assessed by intravascular ultrasound(IVUS), have not been widely established. The aims of present study were to summarize the clinical, angiographic and IVUS findings of patients with ISR after SES implantation and to elucidate the possible mechanism of ISR after SES implantation by using IVUS.
Between March 2003 and April 2005, SES implantation was performed in 502 patients with 670 lesions at the National Health Insurance Corporation Ilsan Hospital(Goyang-si, Korea), and we performed 8-month follow-up angiography with radial artery access in 356 patients(71%) with 495 lesions(74%) at several outpatient clinics. Of these patients, 27 patients had ISR in 28 lesions(5.6%). When ISR presented on the follow-up angiography, we performed IVUS as possible. All the patients received aspirin(100-200 mg/day) and clopidogrel as an immediate 300 mg loading dose, followed by 75 mg/day for at least 6 months. The prespecified clinical and laboratory data were obtained from the hospital charts and coronary database.
Angiographic analysis was performed with a computer-assisted automated edge-detection algorithm(Phillip INTEGRIS BH 5000, Phillips medical systems, Netherlands) by an independent observer who was unaware of the clinical and IVUS findings. Using the guiding catheter for magnification-calibration, the minimal luminal diameter(MLD) and the diameters of the reference segments were measured before and after stenting and at follow-up from the diastolic frames in a single, matched view that showed the smallest MLD. The in-lesion MLD included the stent as well as the 5 mm margins proximal and distal to the stent. The reference vessel diameter(RVD) was the average of the proximal and distal reference lumen diameters. Angiographic restenosis was defined as a diameter stenosis >50% and this was classified as in-stent restenosis if it was inside the stent, or as edge restenosis if it was located within the 5 mm segments distal or proximal to the stent margins.6) The patterns of angiographic instent restenosis were classified as suggested by Mehran et al.9)
Follow-up IVUS imaging was performed after administrating intracoronary nitroglycerin(150-200 µg) with a motorized automatic transducer pullback system, a commercially available scanner(Boston Scientific/SCIMED, Minneapolis, MN) and incorporating a 30 or 40 MHz rotating transducer. The ultrasound catheter was advanced >10 mm beyond the stented segment into the distal vessel. The transducer was withdrawn at a pullback speed of 0.5 mm/s to a point >10 mm proximal to the stent. The ultrasound studies were recorded on 1/2-in high resolution s-VHS tape or compact disk for both on- and off-line analysis.
Quantitative analyses were performed according to the criteria of the clinical expert consensus document on IVUS, and with using computerized planimetry.10) Quantitative measurements at the stented segment and at the proximal and distal reference segments included the external elastic membrane(EEM), lumen, stent CSA and intimal hyperplasia CSA(stent CSA - lumen CSA). Reference segments were defined as the most normal-looking cross-sections within 10 mm proximal and distal to the lesions. Stent underexpansion was defined as the minimum stent CSA at the ISR site <5.0 mm2 and <4.5 mm2 in the cases of small coronary artery(RVD <2.8 mm).11) Stent fracture was defined as non-visualization of the struts on IVUS at the restenotic segments.6)
The mean duration of angiographic follow-up was 221±58 days. The overall clinical characteristics of the 27 enrolled patients with restenosis are shown in Table 1. The mean lesion length was 36.7±19.0 mm. In 12 lesions(43%), implantation of multiple overlapping stents was required for achieving full coverage of the diffuse long lesion on the index procedure. The other angiographic and procedural findings are shown in Table 2. The follow-up angiographic findings are shown in Table 3. The ISR pattern was focal in 26 lesions and diffuse ISR was noted in 2 lesions.
The follow-up IVUS data was obtained for 21 of 28 ISR lesions. Follow-up IVUS study was not performed for the other 7 lesions at the follow-up angiogram because of vasospasm that was due to radial artery access (n=3), inability to pass the guide-wire in the totally occluded restenotic lesion(n=1), and refusal of the test (n=3). Table 4 shows the IVUS measurements of the stented segments with ISR. For the 21 IVUS-applicable lesions, the stent CSA at the ISR site was <5.0 mm2 in 11(52%) lesions. Of the subset of small coronary arteries(RVD <2.8 mm, a total of 8 lesions), the stent CSA at the ISR site was <4.5 mm2 in 7(88%) lesions. Overall stent underexpansion was observed in 10(48%) of the 21 IVUS applicable ISR lesions. Edge stenosis that was located in the proximal edge was noted in 3 lesions(14%) on the follow-up IVUS. Reviewing the index procedural angiogram, one lesion was related to residual dissection after the procedure, and the others were related to balloon trauma outside the stent. Stent fracture was observed in 3 ISR lesions (14%). De nole lesions of 2 ISR lesions were severely angulated lesions, and one ISR lesion was located in the gap between two overlapped stents and this lesion was not fully covered by the stents. The examples of stent underexpansion and stent fracture in the patients with ISR are shown in Fig. 1, 2., respectively.
To examine the possible mechanical causes, we summarized the 21 IVUS-applicable patients with ISR after SES implantation in Table 5. Stent underexpansion was the most common IVUS findings, and this was observed in about half of the ISR cases [10(48%) out of 21 ISR lesions]. Except for edge stenosis, stent underexpansion was observed in 55% of the intra-stent restenotic lesions. Stent underexpansion was the more prominent finding in the small coronary arteries, and this was observed in 7 out of 8 small coronary arteries. There were 7 lesions(33%) in which profound intimal hyperplasia within the stent occurred that was not related to stent underexpansion, stent fracture or edge stenosis. Five of these 7 lesions were diffuse long lesions that required implanting 2 stents.
The main findings of our observational study were: (1) the common IVUS findings of the patients with ISR after SES implantation were stent underexpansion, edge stenosis and stent fracture; (2) stent underexpansion was the most prominent finding, especially in the intra-stent ISR and small coronary arteries; and (3) profound intimal hyperplasia within the stent was observed in one third of the lesions.
In the SIRIUS2) and TAXUS-II trials,12) the restenotic lesions were frequently located near the stent margins or at the site of a gap between stents. Thereafter, the new concept, "the longer, the better" became a widespread strategy for coronary intervention in the DES era. However, recent studies have suggested that the mechanical factors, especially minimal stent CSA, still remain the important predictors of ISR.11)13)14) Sonoda et al.11) reported that the optimal minimum stent CSA threshold for SES to predict ISR was 5.0 mm. In addition, Takebayashi et al.13) have reported that stent underexpansion was observed in 67% of ISR. Hong et al.14) have recently reported that the post-procedural minimum stent CSA(<5.5 mm2), as determined by IVUS, was the independent predictor of ISR after SES implantation.14) In our study, the most common IVUS finding in the restenotic lesions was stent underexpansion. Since stent underexpansion usually occurred in the intra-stent and small coronary arteries, we can speculate that stent underexpansion may be the main cause of ISR, especially in the small coronary arteries and intra-stent lesion.
SES fractures have also been recently reported in cases of focal ISR.15-19) The clinical significance of these findings are currently unknown. In our ISR cases, stent fracture associated with ISR was noted in a minority of ISR lesions(3 out of 21 lesions). The mechanism and clinical significance of SES fracture should be investigated in the future.
The mechanism of edge stenosis is different from those of stent underexpansion and fracture.20) The results of the SIRIUS study and observations by other authors have suggested that ISR, especially edge stenosis, were associated with insufficient lesion coverage and edge damage.5)6) In our study, there were three cases (14%) of edge stenosis on the follow-up angiograms, and they were associated with what was previously mentioned. With employing the stent placement strategy to cover the treated segment "from healthy tissue to healthy tissue", the incidence of this phenomenon has decreased, from 5.7% in the SIRIUS study2) to 3.4% in the E-SIRIUS study,3) and 1.6% in the RESEARCH study.4)
Stent underexpansion, stent fracture or edge restenosis were not related to 7 ISR lesions(33%) in this study. The clinical characteristics of these patients were not very much different from those of the other patients with restenosis; there were 3 cases of diabetes, 3 cases of hyperlipidemia and 3 males among these patients. In addition, there were no small coronary arteries in this subset. Notably, implantation of multiple overlapping stents was required in 5 of 7 patients. Because this subset had no definite risk factors in this analysis, except for long lesion, further evaluation of this subset will be required to understand the ISR of SES.
This study has several limitations. This was a small observational study and pre-intervention IVUS was not performed in all patients. Follow-up IVUS was not performed in all the patients with ISR after SES implantation. Because there was no control group to compare with the ISR group, it was difficult to definitely explain the possible causes of ISR after SES implantation.
IVUS is a useful tool for evaluating the lesions of intra-stent(e. g., a precise description of stent morphology), stent expansion and the relationship of the stent and the vessel wall. The mechanical factors on IVUS, such as stent expansion, stent fracture or non uniform strut distribution, have been in the spotlight more often during the DES era than the systemic factors and they will be investigated in future studies. However, all the ISR lesions were not explained by the mechanical factors. To reduce the incidence of ISR after SES implantation and to understand it, further studies about mechanical factors together with the other systemic factors will be required.
Figures and Tables
Fig. 1
In-stent restenosis caused by stent underexpansion after sirolimus-eluting stent implantation (SES). The pre-stenting (A) and post-stenting angiogram (B) are shown [overlapped with a SES 3.5×33 mm (dashed line) and a SES 2.75×33 mm (dotted line)]. Follow-up angiography (C) demonstrates 90% stenosis in mid-portion of the distal stent of the mid-left anterior descending artery (LAD). IVUS images are from 10 mm proximally (D), the minimal lumen site (E), and 10 mm distally (F) at follow-up. IVUS demonstrated stent underexpansion (minimal stent CSA=4.0 mm2) at the minimum lumen site.
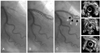
Fig. 2
In-stent restenosis caused by stent fracture after implantation of sirolimus-eluting stent. Pre-stenting (A: left anterior oblique view. B: right anterior oblique view) and the poststenting angiogram (C) after implanting a 2.75×33 mm sirolimus-eluting stent are shown. Follow-up angiography (D) demonstrates 85% stenosis in the proximal portion of the right coronary artery: an arrow indicates the image slice of intravascular ultrasound (IVUS). Stent separation (arrowhead) of mid-portion of the sirolimus-eluting stent was noted on fluoroscopy (lower box in D). The follow-up IVUS images are 5 mm (E) and 2mm (F) proximal to the minimal lumen site (G), and 2 mm (H) and 5 mm (I) distal to the minimal lumen site. An IVUS investigation at the spot of the in-stent restenosis represents non-visualization of struts (G).
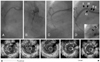
Table 1
Baseline clinical characteristics of 27 patients with in-stent restenosis after sirolimus-eluting stents implantation

Table 2
Baseline angiographic and procedural characteristics of 28 lesions with in-stent restenosis after sirolimus-eluting stents implantation at the index procedure

Table 3
Angiographic analysis of 28 lesions with in-stent restenosis after sirolimus-eluting stents implantation on the follow-up angiogram

References
1. Morice MC, Serruys PW, Sousa JE, et al. Randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002. 346:1773–1780.
2. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003. 349:1315–1323.
3. Schofer J, Schluter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double blind, randomized controlled trial (E-SIRIUS). Lancet. 2003. 362:1093–1099.
4. Lemos PA, Hoye A, Goedhart D, et al. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study. Circulation. 2004. 109:1366–1370.
5. Colombo A, Orlic D, Stankovic G, et al. Preliminary observations regarding angiographic pattern of restenosis after rapamycin-eluting stent implantation. Circulation. 2003. 107:2178–2180.
6. Lemos PA, Saia F, Ligthart JM, et al. Coronary restenosis after sirolimus-eluting stent implantation: morphological description and mechanistic analysis from a consecutive series of cases. Circulation. 2003. 108:257–260.
7. Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet. 2003. 361:247–249.
8. Popma JJ, Leon MB, Moses JW, et al. Quantitative assessment of angiographic restenosis after sirolimus-eluting stent implantation in native coronary arteries. Circulation. 2004. 110:3773–3780.
9. Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999. 100:1872–1878.
10. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS): a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2001. 37:1478–1492.
11. Sonoda S, Morino Y, Ako J, et al. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the SIRIUS trial. J Am Coll Cardiol. 2004. 43:1959–1963.
12. Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003. 108:788–794.
13. Takebayashi H, Kobayashi Y, Mintz GS, et al. Intravascular ultrasound assessment of lesions with target vessel failure after sirolimus-eluting stent implantation. Am J Cardiol. 2005. 95:498–502.
14. Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006. 27:1305–1310.
15. Sianos G, Hofma S, Ligthart JM, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv. 2004. 61:111–116.
16. Takebayashi H, Mintz GS, Carlier SG, et al. Nonuniform strut distribution correlates with more neointimal hyperplasia after sirolimus-eluting stent implantation. Circulation. 2004. 110:3430–3434.
17. Bae JH, Hyun DW, Kim KY, Yoon HJ, Nakamura S. Drug-eluting stent strut fracture as a cause of restenosis. Korean Circ J. 2005. 35:787–789.
18. Min PK, Yoon YW, Moon Kwon H. Delayed strut fracture of sirolimus-eluting stent: a significant problem or an occasional observation? Int J Cardiol. 2006. 106:404–406.
19. Kim JS, Yoon YW, Hong BK, et al. Delayed stent fracture after successful sirolimus-eluting stent (Cypher(r)) implantation. Korean Circ J. 2006. 36:443–449.
20. Berenguer A, Mainar V, Bordes P, Valencia J, Gomez S, Lozano T. Incidence and predictors of restenosis after sirolimus-eluting stent implantation in high-risk patients. Am Heart J. 2005. 150:536–542.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download