Abstract
Background and Objectives
Drug-induced electrocardiographic QT interval prolongation is associated with the occurrence of a potentially lethal form of polymorphic ventricular tachycardia, termed 'torsades de pointes' (TdP). Women are at greater risk for the development of drug-induced TdP. To determine whether this may be the result of gender-specific differences in the effect of quinidine on cardiac repolarization, we compared the degree of quinidine-induced QT interval lengthening in young, healthy volunteers.
Subjects and Methods
Twelve women and 12 men each received a single intravenous dose of quinidine (4 mg/kg) or placebo in a single-blinded, randomized crossover trial. Total plasma concentrations of quinidine were measured, and QT and corrected QT intervals were analyzed.
Results
As expected, the mean QTc interval at baseline was longer for women than for men (443.6±26.9 vs 402.1±31.3 msec, respectively, p=0.037). The mean value of the maximal ΔQTc after quinidine infusion was higher in women (134.4±46.4 vs 117.5±37.7 msec, respectively, p=0.029), and the mean value of the minimal ΔQTc for 1 hour after quinidine infusion was also higher in the female group (47.6±15.7 vs 83.7±25.4 msec, p=0.034). However, there were no significant differences in the time courses of the changes in the quinidine-induced QTc and ΔQTc interval between the two groups (p=0.092, and p=0.305, respectively).
Drugs that prolong cardiac repolarization induce lengthening of the QT interval on electrocardiography (ECG), and are associated with the occurrence of a polymorphic ventricular tachycardia known as torsades de pointes (TdP).1) This term was coined by Dessertenne,2) who observed the characteristic "twisting" of the QRS axis about the isoelectric line during tachycardia. An arrhythmia of this type is usually hemodynamically unstable, and is often clinically manifested as syncope or cardiac arrest.
The incidence is increased by factors such as concomitant hypokalemia, hypomagnesemia, bradycardia, pretreatment QT, and the degree of QT prolongation.1) Makkar et al.3) reported a meta-analysis of 332 published cases of TdP associated with quinidine, disopyramide, amiodarone, sotalol, bepridil, or prenylamine. The authors observed that reports in women were over-represented, which suggested that women are at greater risk of TdP. However, the mechanism by which gender might confer increased risk of TdP is not yet understood. One possible explanation could be that drug concentrations are higher in women because of their smaller body sizes. However, the plasma concentrations of quinidine did not appear to differ between men and women who have experienced TdP.4) In addition, although there are several active metabolites of quinidine, there were no consistent patterns of metabolites in patients in whom torsades de pointes developed.4) One possible explanation for this is that cardiac repolarization in women is intrinsically more sensitive to the effects of drugs. This is supported by previous studies in isolated rabbit hearts perfused with quinidine5) or erythromycin,6) in which the hearts from female animals showed a greater QT prolongation.
Because of the greater incidence of TdP in women treated with quinidine and other drugs that block potassium channels,3) we tested the hypothesis that Korean women have larger increases in QT intervals than men at equivalent serum concentrations. In this study, we used a prospective, randomized crossover design to administer quinidine to men and women, and to compare the relationship between serum quinidine concentration and the corrected QT interval (QTc) prolongation between the sexes. The intravenous route was chosen for this study to minimize the possible confounding effects of active metabolites. To the best of our knowledge, this is the first direct comparison of the pharmacokinetic and pharmacodynamic effects of quinidine between Korean men and women.
Twenty-four healthy Korean volunteers (12 men and 12 women) between 20 and 27 years of age gave written informed consent to participate in this study. This study protocol was approved by the Institutional Review Boards of Busan Paik Hospital. There were no clinically significant abnormalities in any of the subjects' medical histories, physical or mental examinations, blood chemistries, hematological tests, or ECGs. None of the subjects had any signs of arrhythmia, unidentified syncope, ischemic heart disease, valvular heart disease, or myocardial disease in their past or current history of heart disease, nor did they have any general diseases such as diabetes and hypertension. Those who had a QTc interval of more than 460 msec, or any past history of a sensitive response to quinidine were excluded. A negative result from a urine pregnancy test was required for each female subject. All women had regular menstrual cycles, and none of the female subjects was taking oral contraceptives. Subjects ate their usual diet, but were asked to refrain from drinking alcohol, grapefruit juice, and caffeine-containing beverages for 3 weeks before and during the period of the study.
The study was a prospective, randomized, single-blinded, crossover study with a month washout period between the administration of quinidine and placebo. All subjects were administered a single intravenous dose of quinidine gluconate (4 mg/kg of base, Eli Lilly, Indianapolis, IN) or matching intravenous placebo (saline). The quinidine gluconate dose was based on the body weight of each subject, and was diluted to a total volume of 20 mL with normal saline and infused over 20 min with a Harvard infusion pump (Harvard Apparatus Inc, Holliston, MA). Each subject received the infusion at 8 AM after fasting overnight. A 20-gauge intravenous angiocatheter was inserted into each arm of each subject; one was used for the infusion of intravenous quinidine or placebo, and the other was used for the drawing of blood samples. Each subject remained in a supine position in bed for the first 4 hours after the administration of drug or placebo, after which each subject ate a light standardized lunch and was allowed to walk around the study room.
Blood samples were repeatedly drawn from the arm opposite the infusion site at 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, and 55 minutes and at 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, and 24 hours after the administration of quinidine or placebo. Plasma was separated and kept frozen at -20℃ until analysis. Before each blood sample was taken, a 12-lead ECG was recorded on paper and on a computer diskette with a MacVu ECG machine (Marquette Electronics, Milwaukee, WI, USA) at Inje University Hospital using a paper speed of 50 mm/sec. For the measurement of baseline QTc, ECGs were recorded three times within 10 min before the infusion of quinidine or placebo, and each subject was asked to remain supine in bed for 15 min before each ECG.
A second clinical trial using quinidine or placebo was performed with exactly the same protocol as described above, 1 month after the first study. All ECG leads were applied to the same chest and limb sites as in the first study. For the female subjects, the study day was scheduled to be within 5 days after the cessation of menstruation in order to minimize any possible potential contribution of the menstrual cycle to gender differences or to the pharmacological mechanism of quinidine, and to ensure that subjects were not pregnant.
For safety, a cardiac defibrillator, MgSO4, and intubation kit were prepared in the room of the clinical research center, and a cardiologist was always ready in case of cardiac emergency. The subjects were required to stay at the clinical center for up to 24 hours if their QTc interval was longer than 450 msec or longer than the baseline QTc value. If the systolic pressure and diastolic pressure of a subject were lower than 90 mmHg and 60 mmHg, respectively, intravenous quinidine infusion was stopped immediately.
The sampled blood was stored in plain Vacutainer tubes (Becton-Dickinson, Franklin Lakes, NJ) containing heparin (50 unit/mL), and it was centrifuged at 10,000 g for 15 minutes to separate plasma that was stored at -70℃ until it was assayed. A modified solid-phase extraction was used to prepare the samples for quantification of quinidine and 3-hydroxyquinidine by automated high-performance liquid chromatography (HPLC).7)8) In brief, plasma (0.5 mL) was added to a 10 mL glass tube containing an internal standard (5 ng quinine), 0.5 mL of 0.1 m NaOH, and 3 mL of methylene chloride. After vigorous stirring for 3 minutes on a vortex mixer, the aqueous phase was separated by centrifugation (1,000 g for 10 min) and discarded. The remaining organic phase was subsequently evaporated to dryness in a vacuum centrifuge and then reconstituted with 100 mL of mobile phase. Subsequent chromatographic separation was performed on a reversephase column (LiChrospher RP-18, 250×3.9 mm, 5 µm particle size; Merck Co. Darmstadt, Germany) with an isocratic mobile phase consisting of acetonitrile and water (9 : 1, including 0.3% triethylamine, pH 2.5). The flow speed of the mobile phase was 0.6 mL/min. Chromatograms were obtained using the fluorescence detector at an excitation wavelength of 340 nm and an emission wavelength of 425 nm. Extraction recoveries were within the range of 95.2-98.4% for quinidine. The lower limit of quantification was 50 ng/mL, and the coefficients of variation were 2.5% at the lowest quantifiable concentration on the standard curves for quinidine.
ECG changes were measured to record QT intervals and RR intervals using the Hewlett Packard-Page Writer 200/200I ECG recorder (model: HPM1771). The QT interval was gauged by a trained researcher who did not know about the measuring time and medication the subjects had taken. At this time, the QT interval was first measured at V2 lead, and if a U wave was generated at this lead, the longest QT interval of the measurable leads was found and quantified (Fig. 1).
The QTc interval at the associated time was assessed according to the Bazett's method (QTc=QT/RR½).9) The change in QTc (ΔQTc) was determined by subtracting the value of the QTc interval while subjects were receiving placebo from the value of the QTc interval at the identical time point when the subjects were receiving quinidine.
Pharmacokinetic parameters of individual subjects were calculated by noncompartmental analysis using the WinNonlin® program (Pharsight, Cary, NC). The peak concentration (Cmax) of quinidine was taken directly from the measured value, and the peak time (Tmax) until Cmax was also directly measured. The area under the concentration-time curve (AUC 0 → 12 hr) was calculated using a numerical integration method and extrapolated to infinity for AUC (0 → ∞). The clearance (CL) of quinidine was determined as Dose/AUC (0 → ∞). The elimination rate constant (λz) was calculated from the terminal phase of the quinidine concentration-time profile. The steady state volume of the distribution (Vss) was estimated as CL /λz.
Pharmacokinetic parameters and baseline heart rates and QTc intervals were analyzed using an unpaired t-test. One-way analysis of variance (ANOVA) with repeated measurements was performed to verify the statistical significance of the serial quinidine-induced QT prolongation and serum quinidine concentration between the two groups. Continuous variables were summarized as means±SDs, and data analysis was carried out using the SPSS software package, version 13.0 (SPSS, Chicago, IL). Since it was reported that the effect of QTc prolongation changes depending on sex, an analysis was conducted by sex, and a comparison of concentration and ΔQTc with time after intravenous quinidine injection was also conducted. P of less than 0.05 were considered statistically significant.
Twenty-four subjects participated in the study, and none of the subjects experienced any adverse reactions. There were no significant differences in demographics between genders, but the average weight of the men was greater than that of the women (Table 1). All clinical laboratory results from all subjects were within the normal range, and were similar to each other.
There were no gender-related differences in the maximal plasma quinidine concentration (Cmax) (2.63±0.66 vs 2.35±0.79 µg/mL; p=0.187). Pharmacokinetic parameters, including the area under the concentration-time curve (AUC), the quinidine clearance, and the estimated volume of distribution at steady state, were not statistically significant between the men and women. There was no statistical difference in the baseline heart rate between the women (66±4 beats/min) and the men (64±6 beats/min). As estimated, however, the mean baseline QTc interval before quinidine injection was approximately 10% higher in the women than in the men (402.1±31.3 vs. 443.6±26.9 msec, respectively; p=0.037) (Table 2).
Fig. 2 shows the average plasma quinidine concentration-time profiles obtained after the 20 minute infusion of quinidine in the male and female volunteers. After reaching a peak at the end of the infusion, the curve declined rapidly within 15 min, followed by a slow decline. Although the mean concentrations of quinidine in the men tended to be higher than those in the women, this difference was not statistically significant (p=0.127) (Fig. 2).
The time courses of the quinidine-induced QT prolongation in both groups are illustrated in Fig. 3. The QTc interval was markedly prolonged in both groups after intravenous quinidine infusion compared with the QTc interval following saline infusion.
The relationships between quinidine concentration and QTc prolongation in the men and women are illustrated in Fig. 4. The changes in the QTc and ΔQTc profiles were generally paralleled by plasma quinidine concentrations. As shown in Table 2, however, the mean value of the maximal QTc interval in each subject was significantly higher in the women (519.5±47.9 vs 577.4±56.3 msec, respectively; p=0.014). Likewise, the mean value of the maximal ΔQTc was higher in the female group (117.5±37.7 vs 134.4±46.4 msec; p=0.029). The mean value of the minimal ΔQTc for 1hr after intravenous quinidine infusion was higher in the women (47.6±15.7 vs 83.7±25.4 msec; p=0.034).
However, there were no significant differences in terms of the time courses of the quinidine-induced QTc and ΔQTc interval changes between the two groups (p=0.092 and p=0.305, respectively) (Fig. 5).
Since Mirvis10) first commented in 1985 that QT intervals in normal and abnormal states have distinctive spatial distributions that are consistent with known regional myocardial electrophysiology, the QT interval or QT interval dispersion is accepted as an indicator that determines the electrical instability of the heart. A drug-induced prolongation of the QT interval can lead to sudden death11) in relation to the development of polymorphic ventricular tachycardia, known as TdP. However, such results have been carried out mostly in people in Western countries. Although its frequency is relatively low, the development of TdP caused by drug-induced QT interval prolongation has also been reported in the Republic of Korea.12-14) Therefore, the correlation between the concentration of a drug and a QT interval change and QT interval prolongation in the Korean population is a very important issue.
Quinidine is one of the major medicines that causes prolongation of the QT interval,15) and it has been revealed that females are more vulnerable to drug-induced QT interval prolongation than males.3) Furthermore, quinidine may exert its beneficial effects in Brugada syndrome16) and short QT syndrome,17) which may cause a lethal ventricular arrhythmia. From this clinical point of view, it is important to know the pharmacokinetics and the electrocardiographic changes of quinidine in Koreans, as well as the differences in the parameters between the sexes.
The major finding in this study was that quinidine causes a greater prolongation of cardiac repolarization in Korean women than in Korean men at equivalent serum concentrations. Neither the concentration data for quinidine nor the exposure to quinidine could account for the observed gender-related differences in QTc response. Thus, the effect of quinidine on cardiac repolarization appears to be greater in women than in men. The longer baseline QTc and greater sensitivity in women could be sufficient to induce torsades de pointes, especially if associated with other risk factors for torsades de pointes such as hypokalemia and bradycardia.
Our observations may be related to the inherently longer QTc in women compared to men. The QTc interval is equal in male and female children, but shortens in males at the time of puberty, returning to previous levels in the fifth to sixth decades of life.18) These findings suggest that hormonal influences on either the autonomic nervous system or on the expression or activity of cardiac ion channels contribute to gender-related differences in cardiac repolarization, and may also contribute to the greater susceptibility to torsades de pointes in women. It is also possible that the difference in the QT response to erythromycin in male and female rabbit hearts is the result of the lower density of repolarizing potassium currents in female hearts or to the differences in the density of repolarizing potassium currents in male and female rabbit hearts.19)
Data from previous studies in isolated rabbit hearts suggest that a hormonally-based mechanism may contribute to the gender-specific effects of quinidine on repolarization.20) Quinidine caused a smaller increase in the QT interval in the hearts of ovariectomized rabbits treated with dihydrotestosterone than in those treated with estradiol.20) In that same study, sex hormones affected the levels of messenger ribonucleic acid for potassium channels in cardiac tissue. Another possible explanation in this animal model20) is that hormones may have a direct modulating effect on the permeability or gating function of ion channels. Although very high concentrations of estradiol shortened repolarization in atrial tissue,21) there was no apparent acute effect on repolarization when physiological concentrations of estradiol or dihydrotestosterone were added to beating heart preparations.22) This finding supports the hypothesis that estrogens and androgens differentially affect the expression and activity of potassium channels in cardiac tissue. In addition, it has been observed that a long QT and a lower density of potassium channels were present in cardiac cells isolated from female rabbits,19)22) a difference that could contribute to the longer repolarization times and greater absolute QT prolongation upon treatment with drugs that block these channels.
Although this study shows that there is greater QT prolongation in women than in men at similar drug concentrations, suggesting intrinsic differences in cardiac sensitivity to quinidine, an alternative explanation for this is that intracellular concentrations of quinidine may be higher in women. This may be a relevant possibility, since the binding site for quinidine is thought to exist on the inner side of the potassium channel pore.23) Because we only measured serum concentrations, this is a topic for further exploration in in vitro systems. Importantly, such a difference in cellular accumulation would not functionally alter the clinical significance of our observations.
It is not yet known whether the greater drug sensitivity observed in women is specific to quinidine. Greater prolongation of repolarization in women has been observed with d-sotalol at equivalent doses; however, plasma concentrations were not determined.24) The Survival With Oral d-Sotalol (SWORD) trial, which compared d-sotalol with placebo in patients with prior myocardial infarction, was terminated early because of an increased mortality rate in the patients treated with d-sotalol.25) Notably, female sex was a major risk factor for excess mortality in the d-sotalol treatment group.
In addition to its association with antiarrhythmic agents, torsades de pointes is increasingly recognized to be caused by drugs not previously thought to have cardiac effects. For example, terfenadine,14) an antihistamine, cisapride,12) a gastric promotility agent, and erythromycin,26) an antibiotic, have been shown to cause torsades de pointes under certain circumstances.26)27) These agents block potassium channels and cause prolongation of repolarization, a prerequisite for drug-induced torsades de pointes.1) Female sex is a risk factor for QT prolongation and torsades de pointes caused by antiarrhythmic drugs,3)19)27) probucol (an antihyperlipidemic agent),29) and erythromycin (an antibiotic).26) Additional research is needed to determine whether the effects we noted are a general characteristic of all drugs that prolong repolarization.
The implications of this research are that differences between men and women in response to quinidine may be attributable to the actions of hormones. However, the influences of other factors on repolarization, such as the autonomic nervous system, may also play important roles. We attempted to limit the influence of such factors in our observations by including an identical placebo day to account for any circadian effects on QT and for the general stress of the protocol caused by the insertion of intravenous lines. In addition, steady-state dosing regimens may yield different results. Le Coz et al.30) showed that the QT-prolonging effect of sotalol is lower after 2 days of therapy than after the first dose. This might be explained by down-regulation of potassium channels or changes in autonomic tone caused by the drug.
In summary, at equivalent serum concentrations, quinidine prolonged cardiac repolarization to a greater extent in normal adult women than in normal adult men. Our study suggests that the Korean female predisposition to drug-induced torsades de pointes may be, at least partially, attributable to a difference in sensitivity to the effect of quinidine on cardiac repolarization, and probably is not caused by differences in dosage or serum concentrations between men and women. A more complete understanding of the electrophysiological differences between men and women may lead to a better understanding of the mechanism of torsades de pointes and allow identification of means to modify the risks of drugs that prolong repolarization. Our results suggest that it may be important to examine the effects of other steroid hormones (eg, progesterone), pregnancy, the menstrual cycle, and menopause on the response to drugs that have the potential to cause torsades de pointes.
Figures and Tables
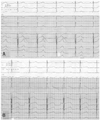 | Fig. 1Representative electrocardiography before (A) and after (B) intravenous quinidine infusion, and QT interval measurement. |
 | Fig. 2Mean plasma quinidine concentration-time curve. Mean plasma quinidine concentration-time profile after intravenous infusion of quinidine (4 mg/kg) for 20 minutes in the 12 male and 12 female healthy Korean subjects. After reaching a peak at the end of the infusion, the curve declined rapidly within 15 min, followed by a slow decline. Although mean concentrations of quinidine in the men tended to be higher than those in the women, this difference was not statistically significant (p=0.127). |
 | Fig. 3Mean QTc interval-time curve with quinidine or placebo. The QTc interval was markedly prolonged in both groups after intravenous quinidine infusion compared with the QTc interval caused by saline infusion. QTc: corrected QT interval. |
 | Fig. 4Mean QTc and ΔQTc interval-time curve according to different plasma quinidine concentrations. The changes in the QTc and ΔQTc profiles were generally paralleled by plasma quinidine concentrations. QTc: corrected QT interval, ΔQTc: delta changes of corrected QT interval. |
 | Fig. 5Mean QTc and ΔQTc interval-time curve with quinidine. There were no significant differences in terms of the time courses of the quinidine-induced QTc and ΔQTc interval changes between the men and women (p=0.092 and p=0.305, respectively). QTc: corrected QT interval, ΔQTc: delta changes of corrected QT interval. |
Table 2
Pharmacokinetic parameters after intravenous infusion of quinidine (4 mg/kg) for 20 minutes, and pharmacodynamic parameters of quinidine-induced QTc interval prolongation
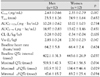
Cmax: maximal plasma quinidine concentration, Tmax: time until maximal plasma quinidine concentration, AUC0→12hr and AUC0→∞: area under the concentration-time curve from 0 to 12 hr and infinity, CL: total clearance, Vss: volume of distribution at steady state, QTc: corrected QT interval, ΔQTc: delta changes of corrected QT interval, minimal ΔQTc: mean value of the minimal ΔQTc for 1 hr after intravenous quinidine infusion.
References
1. Napolitano C, Priori SG, Schwartz PJ. Torsade de pointes: mechanisms and management. Drugs. 1994. 47:51–65.
2. Dessertenne F. Ventricular tachycardia with 2 variable opposing foci. Arch Mal Coeur Vaiss. 1966. 59:263–272.
3. Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993. 270:2590–2597.
4. Thompson KA, Murray JJ, Blair IA, Woosley RL, Roden DM. Plasma concentrations of quinidine, its major metabolites, and dihydroquinidine in patients with torsades de pointes. Clin Pharmacol Ther. 1988. 43:636–642.
5. Drici MD, Burklow TR, Wang WX, Chen Y, Glazer R, Woosley R. Sex steroid hormonal influences upon QT response to quinidine. Circulation. 1995. 92:Suppl. I434.
6. Drici MD, Knollmann BC, Wang WX, Woosley RL. Cardiac actions of erythromycin: influence of female sex. JAMA. 1998. 280:1774–1776.
7. Brandsteterova E, Romanova D, Kralikova D, Bozekova L, Kriska M. Automatic solid-phase extraction determination and high-performance luquid chromatographic determination of quinidine in plasma. J Chromatogr A. 1994. 665:101–104.
8. Nielson F, Kramer K, Brsen K. Determination of quinidine, dihydroquinidine, (3S)-hydroxyquinidine and quinidine N-oxide in plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994. 660:103–110.
9. Bazett HC. An analysis of the times relations of electrocardiograms. Heart. 1918. 7:353–370.
10. Mirvis DM. Spatial variation of the QT intervals in normal persons and patients with acute myocardial infarction. J Am Coll Cardiol. 1985. 5:625–631.
11. Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiogram is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991. 83:1888–1894.
12. Kim JY, Rhee YM, Ahn SK, Lee MH, Kim SS. A case of torsades de pointes induced by cisapride. Korean Circ J. 1999. 29:994–998.
13. Kim SK, Jeon JW, Kim CH, Lee SW, Choi TM, Kwon YJ. Two cases of torsades de pointes after astemizole overdose. Korean Circ J. 1996. 26:593–597.
14. Oh SK, Kuk H, Lim SB, Jeong JW, Park YK, Park OK. Torsades de pointes after treatment with terfenadine and ketoconazole. Korean Circ J. 1998. 28:458–462.
15. Roden DM, Woosley RL, Primm RK. Incidence and clinical features of the quinidine-associated long QT syndrome: implications for patient care. Am Heart J. 1986. 111:1088–1093.
16. Belhassen B, Viskin S, Antzelevitch C. The Brugada syndrome: is an implantable cardioverter defibrillator the only therapeutic option? Pacing Clin Electrophysiol. 2002. 25:1634–1640.
17. Schimpf R, Wolpert C, Gaita F, Giustetto C, Borggrefe M. Short QT syndrome. Cardiovasc Res. 2005. 67:357–366.
18. Rautaharju PM, Zhou SH, Wong S, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992. 8:690–695.
19. Liu XK, Katchman A, Drici MD, et al. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther. 1998. 285:672–679.
20. Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996. 94:1471–1474.
21. de Beer EL, Keizer HA. Direct action of estradiol-17 beta on the atrial action potential. Steroids. 1982. 40:223–231.
22. Knollmann B, Ender SI, Franz MR, Woosley RL. Acute effects of 17-β-estradiol and dihydrotestosterone on action potential duration and QT-interval in isolated rabbit hearts. J Invest Med. 1996. 44:209A. Abstract.
23. Yeola SW, Rich TC, Uebele VN, Tamkun MM, Snyders DJ. Molecular analysis of a binding site for quinidine in a human cardiac delayed rectifier K+ channel: role of S6 in antiarrhythmic drug binding. Circ Res. 1996. 78:1105–1114.
24. Lehmann MH, Hardy S, Archibald D, Quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d.l-sotalol. Circulation. 1996. 94:2535–2541.
25. Waldo AL, Camm AJ, deRuyter H, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. Lancet. 1996. 348:7–12.
26. Drici MD, Knollmann BC, Wang WX, Woosley RL. Cardiac actions of erythromycin: influence of female sex. JAMA. 1998. 280:1774–1776.
27. Antzelevitch C, Sun ZQ, Zhang ZQ, Yan GX. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J Am Coll Cardiol. 1996. 28:1836–1848.
28. Lehmann MH, Hardy S, Archibald D, MacNeil DJ. JTc prolongation with d, l-sotalol in women versus men. Am J Cardiol. 1999. 83:354–359.
29. Reinoehl J, Frankovich D, Machado C, et al. Probucol-associated tachyarrhythmic events and QT prolongation: importance of gender. Am Heart J. 1996. 131:1184–1191.
30. Le Coz F, Funck-Brentano C, Poirier JM, Kibleur Y, Mazoit FX, Jaillon P. Prediction of sotalol-induced maximum steady-state QTc prolongation from single-dose administration in healthy volunteers. Clin Pharmacol Ther. 1992. 52:417–426.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download