Abstract
Background and Objectives
There is increasing evidence to suggest that trimetazidine (TMZ) has the ability to improve ischemic heart failure by way of optimizing the heart's energy metabolism. The aim of this study was to examine the changes of the myocardial enhancement pattern by using two-phase, contrast enhanced, ECG-gated, multi-detector computed tomography (MDCT) after the administration of TMZ in a porcine myocardial infarction model.
Subjects and Methods
The porcine myocardial infarction model was created by balloon occlusion of the left anterior descending coronary artery. We randomized the swine into two groups: group I (n=7: aspirin only) and group II (n=7: aspirin plus 1 mg/kg TMZ for 4 weeks). Echocardiography and MDCT were performed and the ejection fraction (EF, %), end-systolic volume (ESV, mL) and end-diastolic volume (EDV, mL) were measured at 28 days after induction of myocardial infarction. Three enhancement patterns, including the early arterial phase defect (ED), the 4-min late enhancement (LE) and the residual defect (RD), were also investigated and those were described as class I [ED (-), RD (-), LE (±)], class II [ED (+), RD (-), LE (+)], and class III [ED (+), RD (+), LE (+)]. We performed histopathologic examination after sacrificing the animals.
Results
The baseline and follow-up echocardiography at 4 weeks after the induction of MI demonstrated no significant differences between the two groups. The LV indices by MDCT were also similar between the two groups (group I: EF, ESV and EDV=46.0±12.5%, 35.9±23.0 mL and 69.0±40.2 mL, respectively, group II: EF, ESV and EDV=49.8±13.2%, 43.8±23.1 mL and 82.8±24.6 mL, respectively, p=NS). The percent wall thickness was similar (69.1±19.6% vs. 64.9±10.5%, respectively, p=NS), but the enhancement pattern was different between the two groups (group I: class I, II and III=0 (0%), 0 (0%): and 7 (100%) respectively, group II: class I, II and III=0 (0%), 2 (28.6%) and 5 (71.4%), respectively, p<0.001). The volume of tissue that lacked triphenyl tetrazolium chloride was similar between two groups (8.4±1.9% vs. 7.3±2.6%, respectively, p=NS).
Conclusion
TMZ administration produced different enhancement patterns on MDCT. This result suggests that TMZ administration can reduce the residual defect in a porcine myocardial infarction model. Although further experiments are needed for determining the effect of TMZ on reducing the irreversible area of infarcted myocardium, this is the first report that proved the beneficial effect of TMZ by performing MDCT.
Considerable progress has been made over the last 30 years in expanding the available therapeutic options for ischemic heart disease, in terms of both pharmacological and interventional measures that can improve these patients' symptoms and prognosis.1) However, acute coronary syndrome such as myocardial infarction remains one of the leading causes of death in adults, and this is despite the recent advancement in the treatment modalities, including drug-eluting stent and platelet glycoprotein IIb/IIIa receptor blockers.2)
Ischemic heart failure was considered irreversible in past decades, and there was no other treatment option except for hemodynamic agents such as angiotensin converting enzyme inhibitor, beta blocker, diuretics, digoxin and so on.3) But the recent advances in the treatment for ischemic heart failure have demonstrated that ischemic heart failure is reversible.4)
Trimetazidine[1-(2, 3, 4-trimethoxybenzyl) piperazine dihydrochloride, TMZ] is the anti-anginal agent that acts through the optimization of the heart's energy metabolism. Even though no significant negative inotropic or vasodilator properties were shown, several trials have demonstrated the potential benefits of TMZ for treating ischemic heart failure, including improvement of exercise tolerance and reduction of anginal frequency.5-9) Kantor et al.5) suggested that trimetazidine reduces the rate of free fatty acid(FFA) oxidation through the inhibition of enzyme long-chain 3-ketoacyl coenzyme A thiolase, which is a crucial enzyme in the beta oxidation pathway.
Ischemia-induced acceleration of glycolysis and fatty acid oxidation increases the production of protons in the ischemic heart, and this results in intracellular acidosis that's associated with decreased cardiac contractibility.6) The need to use ATP to reestablish H+, Na+, and Ca2+ homeostasis leads to cardiac insufficiency.7) In one experimental study, TMZ inhibited fatty acid oxidation and stimulated glucose oxidation in rat hearts.5)
Moreover, several clinical trials have shown that TMZ has clinical efficacy in patients with various clinical forms of ischemia, including angina pectoris and acute coronary syndrome. However, these studies have usually investigated subjective symptoms such as exercise tolerance and there has been few objective studies concerned with the cardioprotection properties of TMZ.
Two-phase, contrast-enhanced, ECG-gated, multi-detector row computed tomography(MDCT) study is newly emerging technology for evaluating cardiac reversibility because of its ability to show different enhancement patterns. Therefore, in the present study, we evaluated the effects of TMZ on cardioprotection by performing MDCT in a porcine myocardial infarction model.
All the experimental procedures were approved by the Ethics Committee of Chonnam National University Hospital. Pure adult female swine(25 kg each) were provided by and observed in the animal breeding house of Chonnam National University Research Institute of Medical Sciences for 3 to 5 days before the experiment.
All the swine were administrated aspirin(100 mg) daily from 3 days before starting the experiment to the day of sacrifice. We randomized the swine into two groups: group I(n=7: aspirin only), and group II(n=7: aspirin plus 1 mg/kg TMZ for 4 weeks). Following premedication with azaperone(0.5 mg/kg, intramuscular) and xylazine(8 mg/kg, intramuscular), midazolam (0.2 mg/kg, intravenous), normal saline was infused via a 20-G venous access line placed in an ear vein. A 7 Fr. arterial sheath was placed in the left carotid artery after achieving local anesthesia via 2% lidocaine with using the same protocol as was previously reported.10-12) After infusion of 10,000 units of heparin and amiodarone(5 mg/kg, intravenous), a 7 Fr. coronary artery guiding catheter was placed within the ostium of the left coronary artery under fluoroscopic guidance by a mobile C-arm(Phillips BV-25 Gold). During the experiment, oxygen and normal saline were supplied continuously and the anesthesia was maintained with an additional infusion of midazolam. Continuous ECG monitoring was performed to confirm the swine had a normal ST segment at baseline and also ST elevation during the ischemic period. The occurrence of ventricular tachyarrhythmia was also monitored.
Echocardiography(Acuson Cypress) was performed at baseline to evaluate the left ventricle(LV) function, including the left ventricular end-diastolic dimension (LVEDD), the left ventricular end-systolic dimension (LVESD), the fractional shortening(FS) and the left ventricular ejection fraction(LVEF).13)
The myocardial infarction model was created by placement of a balloon(3.0×20 mm, Terumo Co. Tokyo, Japan) just below the first diagonal branch or the first septal branch. Complete occlusion was done by dilating the balloon(up to 6 atm) for 30 minutes.
Each subject was closely observed about one hour for the occurrence of ventricular tachyarrhythmias(ventricular tachycardia or fibrillation). After the experiment, all the swine were transported back to the animal breeding house and then monitored.
After administering each drug for 4 weeks, echocardiography and MDCT were performed after placing the animals under anesthesia with using the same method as the first experiment. Echocardiography was performed just before MDCT scanning at the cardiac CT room. Additional control of the heart rate(HR) was achieved by infusing esmolol HCL(0.5 mg/kg/min, intravenous). Measurement of the non-viable myocardium was performed by two-phase, contrast-enhanced, ECG-gated MDCT and using a 2,3,5-triphenyl tetrazolium chloride(TTC) solution.
The CT examinations were performed with a MDCT scanner(Sensation cardiac 64, Siemens, Forchheim, Germany) set at a 0.75-mm section thickness with a gantry rotation time of 330 msec and a kernel value of B25f. The tube current was 800 mAs at 120 kVP. The pitch, defined as the ratio of the table feed in one rotation over the detector coverage in the transverse direction, was determined as 0.2. Two radiologists, who were blinded to what groups the swine were in and they specialized in reading CT scans, assessed the presence, location and pattern of the myocardial enhancement; the results were reached via consensus of the scan readers. We could not control the HR to less than 100 beats per minute in one swine of group I even after administering multiple drugs, as described above, and we excluded this swine from the data analysis.
Serial CT scanning in the axial plane, together with a ECG triggered examination, was performed from the level of the left ventricular apex after a bolus injection of 60 mL of non-ionic contrast media(Ultravist 370®, Schering) followed by a 60 mL saline bolus injection through an ear vein, and both were injected at a flow rate of 4 mL/sec.14)15) If the target area density was more than 100 Hounsfield units(HU) at the descending thoracic aorta after contrast media injection, then the first scan was done after a 5-sec delay. The scan time was from 13-sec to 15-sec. If any stair step artifact developed, additional scanning was done for further data acquisition.
The second scan was also done after a 4-min delay (Fig. 1). Because there were differences of the myocardial thickness according to the cardiac cycle, we used retrospective electrocardiographic gating at 65% of the RR interval in the workstation(Wizard™, Siemens medical solution) to avoid misinterpretation of any perfusion defect and late enhancement. We analyzed the perfusion defects and enhancement patterns of the infarcted myocardium after a 4-min delay. The three enhancement pattern, including the early arterial phase defect(ED), the 4-min late enhancement(LE) and the residual defect (RD) were investigated and those were described as class I[ED(-), RD(-), LE(±)], class II[ED(+), RD(-), LE(+)], and class III[ED(+), RD(+), LE(+)](Fig. 2, 3).16-21)
The axial images were reconstructed at multiple phases that covered the cardiac cycle in increments of 10% of the RR interval between 5% and 95%. Multiphase reconstruction was performed using short axis slices from the base to the apex of the heart with using commercially available software(Argus®, Siemens). End-diastole and end-systole were defined as the maximal and minimal left ventricular volume, respectively. The end-diastolic volume(EDV), end-systolic volume(ESV) and LVEF after the creation of MI(myocardial infarction) were compared between the two groups(Fig. 4).22)23) The percent wall thickness was defined as the thickness of the thinnest anteroseptal wall divided by the mean thickness of the LV wall at four point.
Immediately after conducting the MDCT examination, the animals were sacrificed and their hearts were extracted. The extracted heart was rinsed and resected at 1 cm thickness by using a microtome and the slices were stained by TTC solution until portions of the viable myocardium turned brick red; these were photographed at 600 dpi with using a commercially available digital camera.
After being fixed in 10% formalin, the myocardial sections were analyzed under Hematoxylin-Eosin(H/E) staining and Masson-Trichrome(M/T) staining(Fig. 5). The sections were reviewed by 2 cardiac pathologists, and they determined the pathological changes at the infarcted area and the normal myocardium.
All data were designated as means±standard deviations and comparisons between the two groups were performed by the nonparametric method with using SPSS for windows, version 12.0(Chicago, Illinois, USA). We used the Mann-Whitney U test for comparing the means, and the binomial test was used for analysis of the categorical data. P values less than 0.05 were considered as significant.
A total 17 animals were enrolled in this study. Ventricular fibrillation(VF) developed in 7 animals during balloon catheter occlusion(Group I: 2(28.6%), Group II: 5(71.4%), p<0.001) and the VF was successfully terminated by performing direct current cardioversion.
We successfully induced the porcine MI model in all the animals, but sudden death within 48 hours after the induction of MI developed in the three swine. We excluded these three swine from the analysis.
The baseline echocardiographic findings, including the LVEDD, LVESD, FS and LVEF, demonstrated no significant differences between the 2 groups. The follow-up echocardiographic parameters at 4 weeks after the induction of MI changed significantly, but there were no differences between the two groups(Table 1, 2).
The baseline HR was similar between the 2 groups (76.3±13.3 vs. 73.9±13.4, respectively, p=NS). The MDCT findings for the LV function, including EDV, ESV and LVEF at 4 weeks after the induction of MI, were not different between the 2 groups. The percent wall thickness was similar(69.1±19.6% vs. 64.9±10.5%, respectively, p=NS); however, the enhancement pattern was different(group I: class I, class II and class III=0(0%), 0(0%) and 7(100%), respectively; group II: class I, class II and class III=0(0%), 2(28.6%) and 5(71.4%), respectively, p<0.001)(Table 3).
The volume of the tissue that lacked TTC, which was compatible to the infarcted nonviable myocardium, was similar between the two groups(8.4±1.9% vs. 7.3±2.6%, respectively, p=NS). We also confirmed this infarcted myocardium by performing H/E and M/T staining.
There has been significant technological development of mechanical CT scanners since the year 2000, which is when the first multi-slice CT scanners were introduced. Yet the imaging modalities for the heart require high temporal and spatial resolution for evaluating the constantly moving small coronary arteries. Moreover, to image the heart, every slice of it has to be reconstructed at the same cardiac phase to avoid gaps between the slices. The modern 64-slice CT systems have rotation times of less than 500 ms, and the thin collimation and slices of less than 0.75 mm provide high temporal and spatial resolution, respectively. The phase of the cardiac cycle can be determined with the help of the simultaneously recorded ECG signals.24)25)
Infarcted myocardium had unique pattern; this is the low density seen on the precontrast CT and the hypoenhancement or paradoxical hyperenhancement seen on the delayed enhanced CT.16)17) But there have only been a few reports published regarding the detection of myocardial infarctions with using MDCT. The enhancement pattern observed via MDCT has an emerging role in terms of evaluating cardiac reversibility. When RDs are detected, as in the class III enhancement patterns, then functional recovery will not be observed. However, when the EDs turned into LE, as in the class II enhancement patterns, the deterioration of the left ventricular function would be minimal or even less than that observed in the class III enhancement patterns. So, RDs might correspond to myocardial necrosis with extensive capillary disorder(myocardial necrosis with extensive capillary damage), i.e., microvascular no-reflow. On the other hand, LE might correspond to the muscle layer where the blood supply was preserved to some degree, indicating the possibility of residual myocardial viability.16-19)
Cardiac MRI is the standard for evaluating the delayed enhancement of myocardium, and evaluating the delayed enhancement by MDCT is still under investigational study. There are few reports on the enhancement pattern of MDCT after four weeks of MI. There is controversy about when is proper time to evaluate the delayed enhancement by MDCT between the two groups. However, our results showed that four weeks administration of TMZ produced different enhancement patterns on MDCT. Because we tried to investigate the cardioprotective effect of TMZ, we performed MDCT scanning four weeks after the creation of MI.26-28)
In our study, there were no differences of the echocardiography parameters such as the LV chamber size and LV systolic function between the two groups after 4 weeks follow-up. However, there were two cases of class II enhancement on the MDCT of the TMZ group.
The possible explanation for the increased incidence of class II enhancement in the TMZ group is the changing energy production in the presence of a reduced myocardial oxygen supply. The effects of TMZ on energy metabolism may contribute to the improved myocardial contractile function in ischemic cardiomyopathy. Some studies have confirmed this hypothesis by using echocardiographic and single photon emission computerized tomography(SPECT) techniques. El-Kady et al.29) have demonstrated improvements in the stress and rest perfusion scores, as well as in the systolic wall thickness, after 24 months of TMZ treatment with using SPECT. Belardinelli et al.8) evaluated the contractile response to low-dose infusions of dobutamine at baseline and after 2 months of treatment with TMZ or placebo. At the end of the follow-up period, TMZ significantly improved the rest and peak systolic wall thickening score index, the LVEF and the peak oxygen consumption.
We administered amiodarone before balloon occlusion of the left anterior descending coronary arteries, but there was VF in seven swine. Although further studies are needed, we thought the different incidence of VF between the two groups would not be meaningful because of the relatively good long term prognosis of early primary VF complicating acute myocardial infarction.30)
There are several limitations in our studies. First, our study had relative small study population(n=14) and the duration of study was short(4 weeks) for determining the potential mechanisms by which TMZ may be effective. It is possible that further differences might have been noted if the study had been extended for a longer duration or conversely, that some of the effects may have been attenuated over time. Second, we administered only a standard dose of TMZ(1 mg/kg/day). There was no comparison with a lower or higher dose, so further studies are needed for determining the optimum dose of TMZ. Third, neovascularization by TMZ has not yet been established by basic research and our data did not include any basic research results for myocardial vascularity. Further investigation for the relationship between TMZ and the increased myocardial perfusion is needed. Fourth, the growth of swine was another limitation. The large body weight of the swine after 28 days growth required large amounts of injected contrast media. Because the wash out of the contrast media on the 5-min delay image produced no definitive discrimination between the LV wall and the LV chamber, we used a different scan protocol such as a 4-min delay instead of a 5-min delay.
TMZ administration produced different enhancement patterns on MDCT. This result suggests that TMZ administration can reduce the residual defect in a porcine myocardial infarction model. Though further experiments are needed for determining the effect of TMZ on reducing the irreversible area of infarcted myocardium, this is the first report that has proved the beneficial effect of TMZ by using MDCT. Because there were no effects on blood pressure or heart rate, TMZ can be used, after further investigation and validation, as a potential adjunctive therapy in clinical practice.
Figures and Tables
Fig. 1
Protocol of the two phase contrast enhanced ECG gated multi-detector computed tomography. If the target area density would be more than 100 HU at the descending thoracic aorta after contrast media injection, then the first scan was done after a 5-sec delay. The second scan was also done after a 4-min delay. ECG: electrocardiography, HU: Hounsfield units.

Fig. 2
Illustrations of the three enhancement patterns: Class I, the absence of EDs in the early phase and the presence of LE without RDs in the late phase, Class II, the presence of EDs in the early phase and the presence of LE without RDs in the late phase and Class III, the presence of EDs in the early phase and the presence of both LE and RDs in the late phase. ED: early arterial phase defect, LE: late enhancement, RD: residual defect.

Fig. 3
Class II enhancement pattern (upper panel) and Class III enhancement pattern (lower panel). A, D: there was early defect seen on the early image (arrow head). B: there was late enhancement seen at the 4-min delay (black arrow). C, F: there was infarcted myocardium noted after staining with TTC solution (white arrow). E: there was late enhancement (black arrow) and residual defect (white arrow) seen at the 4-min delay. There were subendocardial infarction (C) and transmural infarction with wall thinning (F) at the left ventricle that was supplied by the left anterior descending coronary arteries. TTC: 2,3,5-triphenyl tetrazolium chloride.
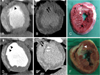
Fig. 4
The end-systolic volume (A), end-diastolic volume (B), and left ventricular ejection fraction were calculated by multiphase reconstruction; this was performed from the base to the apex of the heart with using commercially available software (Argus®, Siemens).
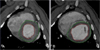
Fig. 5
Hematoxylin (×400)/Eosin (×400)(A) and Masson-Trichrome (B) staining revealed severe fibrosis in the border zone of the infarcted myocardium.

Table 2
Evaluation of left ventricular function by echocardiography between the two groups at 4 weeks

References
1. Lee L, Horowitz J, Frenneaux M. Metabolic manipulation in ischemic heart disease, a novel approach to treatment. Eur Heart J. 2004. 25:634–641.
2. De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial Infarction: a meta-analysis of randomized trials. JAMA. 2005. 293:1759–1765.
3. O'Meara E, McMurray JJ. Myocardial metabolic manipulation: a new therapeutic approach in heart failure? Heart. 2005. 91:131–132.
4. Di Napoli P, Taccardi AA, Barsotti A. Long term cardioprotective action of trimetazidine and potential effect on the inflammatory process in patients with ischaemic dilated cardiomyopathy. Heart. 2005. 91:161–165.
5. Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000. 86:580–588.
6. Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol. 2002. 39:718–725.
7. Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990. 258:C967–C981.
8. Belardinelli R, Purcaro A. Effects of trimetazidine on the contractile response of chronically dysfunctional myocardium to low-dose dobutamine in ischaemic cardiomyopathy. Eur Heart J. 2001. 22:2164–2170.
9. Manchanda SC, Krishnaswani S. Combination treatment with trimetazidine and diltiazem in stable angina pectoris. Heart. 1997. 78:353–357.
10. Rhew JY, Jeong MH, Lee SR, et al. The effects of radiation using Ho-166 on endothelial function in a porcine coronary model. Korean Circ J. 2002. 32:118–124.
11. Kim W, Jeong MH, Hong YJ, et al. A new porcine model of ischemic heart failure and pathologic findings by intra-coronary injection of ethanol. Korean Circ J. 2004. 34:900–908.
12. Lim SY, Jeong MH, Ahn YG, et al. The effect of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Korean Circ J. 2005. 35:734–741.
13. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendation regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978. 58:1072–1083.
14. Hoffmann U, Millea R, Enzweiler C, et al. Acute myocardial infarction: contrast-enhanced multi-detector row CT in a porcine model. Radiology. 2004. 231:697–701.
15. Mahnken AH, Bruners P, Katoh M, Wildberger JE, Gunther RW, Buecker A. Dynamic multi-section CT imaging in acute myocardial infarction: preliminary animal experience. Eur Radiol. 2006. 16:746–752.
16. Koyama Y, Mochizuki T, Higaki J. Computed tomography assessment of myocardial perfusion, viability, and function. J Magn Reson Imaging. 2004. 19:800–815.
17. Koyama Y, Matsuoka H, Mochizuki T, et al. Assessment of reperfused acute myocardial infarction with two-phase contrast-enhanced helical CT: prediction of left ventricular function and wall thickness. Radiology. 2005. 235:804–811.
18. Lardo AC, Cordeiro MA, Silva C, et al. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006. 113:394–404.
19. Mahnken AH, Koos R, Katoh M, et al. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005. 45:2042–2047.
20. Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006. 113:823–833.
21. Ko SM, Seo JB, Hong MK, et al. Myocardial enhancement pattern in patients with acute myocardial infarction on two-phase contrast-enhanced ECG-gated multidetector-row computed tomography. Clin Radiol. 2006. 61:417–422.
22. Heuschmid M, Rothfuss JK, Schroeder S, et al. Assessment of left ventricular myocardial function using 16-slice multidetector-row computed tomography: comparison with magnetic resonance imaging and echocardiography. Eur Radiol. 2006. 16:551–559.
23. Raman SV, Shah M, McCarthy B, Garcia A, Ferketich AK. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am Heart J. 2006. 151:736–744.
24. Nieman K, Cury RC, Ferencik M, et al. Differentiation of recent and chronic myocardial infarction by cardiac computed tomography. Am J Cardiol. 2006. 98:303–308.
25. Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol. 2006. 48:1919–1928.
26. Bertomeu-Gonzalez V, Bouzas-Mosquera A, Kaski JC. Role of trimetazidine in management of ischemic cardiomyopathy. Am J Cardiol. 2006. 98:Suppl. 19J–24J.
27. Danchin N. Clinical benefits of a metabolic approach with trimetazidine in revascularized patients with angina. Am J Cardiol. 2006. 98:8J–13J.
28. Dib N, Diethrich EB, Campbell A, Gahremanpour A, McGarry M, Opie SR. A percutaneous swine model of myocardial infarction. J Pharmacol Toxicol Methods. 2006. 53:256–263.
29. El-Kady T, El-Sabban K, Gabaly M, Sabry A, Abdel-Hady S. Effects of trimetazidine on myocardial perfusion and the contractile response of chronically dysfunctional myocardium in ischemic cardiomyopathy: a 24-month study. Am J Cardiovasc Drugs. 2005. 5:271–278.
30. Volpi A, Cavalli A, Santoro L, Negri E. Incidence and prognosis of early primary ventricular fibrillation in acute myocardial infarction: results of the Gruppo Italiano per lo Studio della Sopravvivenza nell' Infarto Miocardico (GISSI-2) database. Am J Cardiol. 1998. 82:265–271.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download