Abstract
Background and Objectives
This study aimed to assess the effect of simvastatin therapy on plaque regression and vascular remodeling in peristent reference segments of normocholesterolemic patients by using serial intravascular ultrasound (IVUS) observation.
Subjects and Methods
We retrospectively evaluated the poststenting and follow-up IVUS findings in 208 peristent (bare metal stent) reference segments of 108 normocholesterolemic patients (20 mg/day simvastatin group; n=62 vs. non-simvastatin group; n=46); 100 segments were proximal and 108 segments were distal to the stent. Quantitative volumetric IVUS analysis was performed for 5-mm vessel segments immediately proximal and distal to the stent.
Results
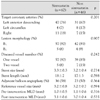
Follow-up IVUS was performed at a mean of 8.7 months after stenting (range: 3-19 months). For the proximal edge, a significant decrease in the mean lumen area and mean external elastic membrane (EEM) area and a significant increase in the mean plaque and media (P&M) area were observed at follow-up in both simvastatin and non-simvastatin groups. However, the changes in EEM (simvastatin: -0.4±0.3 mm2 vs. non-simvastatin: -0.4±0.4 mm2, p=0.983), lumen (simva-statin: -0.7±0.3 mm2 vs. non-simvastatin: -1.0±0.5 mm2, p=0.114), and P&M area (simvastatin: 0.3±0.2 mm2 vs. non-simvastatin: 0.6±0.4 mm2, p=0.110) from poststenting to follow-up at the proximal edge were not significantly different between the 2 groups. For the distal edge, a significant decrease in the mean lumen area and a significant increase in the mean P&M area were observed at follow-up in both the groups. However, the changes in the EEM area (simvastatin: -0.1±0.2 mm2 vs. non-simvastatin: -0.2±0.3 mm2, p=0.674), lumen area (simvastatin: -0.6±0.2 mm2 vs. non-simvastatin: -1.0±0.4 mm2, p=0.087), and P&M area (simvastatin: 0.5±0.2 mm2 vs. non-simvastatin: 0.8±0.3 mm2, p=0.102) from poststenting to follow-up at the distal edge were not significantly different between the groups.
The effects of statins on stent edge and reference segments can be classified into an increase or decrease in the vessel area and/or the plaque area by using intravascular ultrasound (IVUS).1-7) Serial examinations of plaques are particularly important because they may allow insights into the mechanisms involved.
Recent trials have demonstrated that intensive lipid-lowering therapy with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) improved clinical outcomes8) and reduced the progression of atherosclerosis.9) The beneficial effects of statins, other than the lipid-lowering action, mostly depend on their anti-inflammatory properties.10) Simvastatin has also been proved to inhibit smooth muscle cell proliferation.11)
To the best of our knowledge, little data are available about the effects of statins on plaque regression in peristent reference segments, particularly those of normocholesterolemic coronary patients. In the present study, we assessed the effects of simvastatin therapy on plaque regression and vascular remodeling in peristent [bare-metal stent (BMS)] reference segments of normocholesterolemic patients by using serial IVUS.
In this retrospective study, we included 108 normocholesterolemic patients who were treated with BMS implantation under IVUS guidance from January 2004 through December 2004 at Chonnam National University Hospital, Gwangju, Korea. The patients were divided into 2 groups: simvastatin (n=62) and non-simvastatin groups (n=46). In the simvastatin group, therapy with 20 mg/day simvastatin was initiated immediately after stent implantation. All patients in the simvastatin group had taken simvastatin uninterruptedly up to the follow-up period.
Among the 216 lesions, 8 segments proximal to the stent were excluded because of their ostial location. Therefore, 208 peristent reference segments were available for analysis; 100 segments were proximal and 108 segments were distal to the stent. Normocholesterolemia was defined as the serum total cholesterol level of <200 mg/dL, LDL cholesterol level of <130 mg/dL, and triglyceride level of <200 mg/dL.
The exclusion criteria were as follows: narrowing of the left main coronary artery (luminal diameter <50%); triple vessel disease; left ventricular ejection fraction, <40%, hepatic or renal dysfunction (alanine aminotransferase and aspartate aminotransferase levels, >2 times the normal level and creatinine level, >1.5 mg/dL); and current therapy with any lipid-lowering drugs. Hospital records of all the patients were reviewed to obtain information on clinical demographics and medical history.
For all the patients, serum was collected before stent implantation to assess the lipid profile and measure the high-sensitivity C-reactive protein level. All laboratory values were measured after an overnight fast. The serum levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol were measured using standard enzymatic methods. The high-sensitivity C-reactive protein level was analyzed turbidimetrically using sheep antibodies against human C-reactive protein; this method has been validated against the Dade-Behring method.12) The serum levels of total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, and high-sensitivity C-reactive protein were measured at baseline and at 6 months.
The patients were scheduled to undergo elective stent implantation for de novo lesions in native coronary arteries with a diameter between 2.5 and 4.0 mm. Stent implantation was performed as previously described.13) All stenotic lesions were predilated, and stents were deployed at 10-18 atm.
The patients were observed with regard to in-stent restenosis during the 6-month follow-up period. Angiographic restenosis was defined as ≥50% stenosis in the stented segment at follow-up or at least 50% loss of the original gain in the minimal luminal diameter.
Angiograms were analyzed using a validated quantitative coronary angiography (QCA) system (Phillips H5000 or Allura DCI program; Philips Medical Systems, The Netherlands). With the outer diameter of the contrastfilled catheter as the calibration standard, the minimal lumen diameter and reference diameter were measured in diastolic frames from orthogonal projections.
IVUS examinations were performed post-intervention and at follow-up after intra-coronary administration of 200µg nitroglycerin by using a commercially available IVUS system (Endosonics IVUS system; Endosonics Corp., Rancho Cordova, CA, USA), which enables the digital storage of pullback sequences. The IVUS catheter was advanced distal to the target lesion, and imaging was performed with retrograde pullback at an automatic pullback speed of 0.5 mm/s.
We performed IVUS analysis for each 1-mm subsegment and for the entire 5-mm edge segments. Therefore, both proximal and distal vessel segments were further divided into 1-mm subsegments. For each subsegment, external elastic membrane (EEM) and lumen areas were measured, and plaque and media (P&M) area (EEM area minus lumen area) and plaque burden (P&M area divided by EEM area) were calculated from each cross-sectional slice and expressed as the mean values. Area changes (Δ values) for each parameter were calculated as follow-up value minus the poststenting value.
Statistical analysis was performed using the commercially available software (SPSS Version 11). Continuous variables were presented as the mean value±1 SD and compared using paired or unpaired Student's t-test or using the nonparametric Wilcoxon test if the normality assumption was violated. Discrete variables are presented as percentages and relative frequencies; comparisons were conducted using the chi-square test or Fisher's exact test as appropriate. A value of p<0.05 was considered significant.
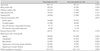
Non significant differences were observed between the 2 groups in patient demographics and other medications (Table 1). At follow-up, the total and LDL cholesterol levels were significantly decreased and the HDL cholesterol level was significantly increased in the simvastatin group but not in the non-simvastatin group. Non significant difference in the high-sensitivity C-reactive protein level was observed between the 2 groups (Table 2).
There were non significant differences in the baseline coronary angiographic and procedural findings (Table 3). At follow-up, binary in-stent restenosis was present in 18% (11/62) and 22% (10/46) of the patients in the simvastatin group and non-simvastatin group, respectively. Repeat revascularization was performed in 15% (9/62) and 17% (8/46) of the patients in the simvastatin group and non-simvastatin group, respectively. However, the differences in these percentages between the 2 groups were not statistically significant.
The follow-up IVUS was performed at a mean of 8.7 months after stenting (range, 3-19 months). Non stent edge dissections were noted post-intervention. Overall, the mean P&M area had increased (proximal edge: 0.5±0.3 mm2, p<0.001 and distal edge: 0.7±0.3 mm2, p<0.001), and the mean EEM area (proximal edge: -0.4±0.3 mm2, p=0.001 and distal edge: -0.2±0.2 mm2, p=0.089) and the mean lumen area (proximal edge: -0.9±0.4 mm2, p<0.001 and distal edge: -0.9±0.3 mm2, p<0.001) had decreased from poststenting to follow-up.
In the case of the proximal edge, the mean lumen area and the mean EEM area had significantly decreased, whereas the mean P&M area had significantly increased at follow-up in both the groups. However, the changes in the EEM area (simvastatin: -0.4±0.3 mm2 vs. non-simvastatin: -0.4±0.4 mm2, p=0.983), lumen area (simvastatin: -0.7±0.3 mm2 vs. non-simvastatin: -1.0±0.5 mm2, p=0.114), and P&M area (simvastatin: 0.3±0.2 mm2 vs. non-simvastatin: 0.6±0.4 mm2, p=0.110) from poststenting to follow-up at the proximal edge were not significantly different between the 2 groups. Regarding the distal edge, although the EEM area had not changed from poststenting to follow-up in both the groups, the mean lumen area had significantly decreased and the mean P&M area had significantly increased at follow-up in the 2 groups. However, the changes in the EEM area (simvastatin: -0.1±0.2 mm2 vs. non-simvastatin: -0.2±0.3 mm2, p=0.674), lumen area (simvastatin: -0.6±0.2 mm2 vs. non-simvastatin; -1.0±0.4 mm2, p=0.087), and P&M area (simvastatin: 0.5±0.2 mm2 vs. non-simvastatin: 0.8±0.3 mm2, p=0.102) from poststenting to follow-up at distal edge were not significantly different between the 2 groups (Table 4) (Fig. 1).
Although the lumen loss in the first 3 mm of the segment was primarily due to the increase in the P&M area rather than the change in the EEM area and the lumen loss beyond the 3-mm portion was due to a combination of increased P&M area and decreased EEM area, there were non significant differences in the changes in the P&M, EEM, and lumen areas at every 1-mm segment between the simvastatin and non-simvastatin groups. Poststenting, the sites with the minimum lumen in the peristent reference segments were located at 3.3±2.0 mm from their respective proximal stent edges and 3.1±1.8 mm from their respective distal stent edges. At these sites, an increase in the P&M area (proximal edge: 0.5±0.3 mm2, p<0.001 and distal edge: 0.6±0.4 mm2, p<0.001) and a decrease in the EEM area (proximal edge: -0.6±0.3 mm2, p<0.001 and distal edge: -0.4±0.3 mm2, p=0.001) and the lumen area (proximal edge: -1.1±0.5 mm2, p<0.001 and distal edge: -1.0±0.4 mm2, p<0.001) were observed from poststenting to follow-up.
In-stent restenosis occurred in 21 patients, including restenosis in 11 stent edges (5 proximal edges and 6 distal edges). The lumen losses accompanied with a greater increase in the P&M area and a greater decrease in the EEM area were more significant in the in-stent resetnosis group than in the no in-stent restenosis group from poststenting to follow-up [EEM area (in-stent restenosis: -0.8±0.5 mm2 vs. no in-stent restenosis: -0.3±0.4 mm2, p=0.001), lumen area (in-stent restenosis: -2.2±1.3 mm2 vs. no in-stent restenosis: -0.8±0.4 mm2, p<0.001), and P&M area (in-stent restenosis: 1.4±0.9 mm2 vs. no in-stent restenosis: 0.5±0.4 mm2, p<0.001)]. The changes in the EEM, lumen, and P&M areas were not significantly different with regard to whether or not poststenting adjunct balloon angioplasty was performed.
The results of this study demonstrate that the conventional dose of simvastatin therapy does not inhibit disease progression (plaque increase and lumen loss) in peristent reference segments of normocholesterolemic patients who have undergone BMS implantation.
The response of the adjacent reference segments not covered by the stent is of a major interest. Several studies have demonstrated lumen loss adjacent to the stent edge after BMS implantation. Hoffmann et al.2) performed serial IVUS analysis at (1) the most normal-appearing cross-sectional area within the 10-mm segment proximal or distal to the stent, (2) midway between this slice, and (3) at the proximal or distal edge of the stent. In this study, the more distant part of the reference segments showed a greater degree of remodeling (decrease in the EEM area) than tissue growth; on the other hand, compared to the further distant reference segments, the anatomic sections sampled at a point closer to the edge of the stent showed a similar amount of remodeling and a greater degree of cellular proliferation (increase in the P&M area). Mudra et al.3) reported absence of relevant progression of the disease adjacent to the stent despite a considerable plaque burden within the reference segments. Weissman et al.4) analyzed the reference segments that were 10 mm proximal and distal to the stent at baseline and follow-up. In this study, the lumen loss was most pronounced within the first 2 mm of the stent edge in the adjacent reference segments and was primarily due to intimal proliferation, whereas the loss beyond 2 mm was attributed to negative remodeling.
Statins inhibit mevalonate synthesis and lower the LDL cholesterol level. Besides lowering lipids, statins have favorable effects on vascular inflammation,14-16) endothelial function,17)18) and platelet adhesion and thrombosis.19) Multiple studies have shown that statins lowered mortality and morbidity in coronary artery disease and other atherosclerotic vascular disease.20-22)
Several studies have demonstrated that statin therapy prevents the progression of coronary atherosclerosis in normocholesterolemic patients. Nakagawa et al.23) reported that cholesterol-lowering pravastatin therapy prevents the progression of coronary atherosclerosis in normocholesterolemic patients with coronary artery disease. Tamura et al.24) reported that this therapy could prevent the progression of coronary atherosclerosis even in normocholesterolemic patients with an established coronary artery disease.
Several IVUS studies demonstrated the effects of statins on plaque regression and vessel remodeling. Suzuki et al.25) reported that plaque area decreased by 12% in patients who received a statin and increased by 13% in those who did not receive a statin. They also reported that vessel area did not enlarge in patients treated with a statin but was positively remodeled in patients with progressive plaque and not treated with a statin. Jensen et al.26) reported a significant reduction in the lesion EEM area by 4.6% and in the lesion plaque area by 5.9%; however, there was no change in the reference measurements obtained 12 months after simvastatin treatment. Hence, the remodeling index was reduced by simvastatin from 1.01±0.12 to 0.95±0.09.
Thus far, only few studies have demonstrated the effects of statins on plaque regression and vessel remodeling in normocholesterolemic patients by using IVUS. Petronio et al.27) reported that a 12-month simvastatin therapy did not prevent intimal hyperplasia, but it promoted atherosclerotic regression both at the stented and nonstented sites in normocholesterolemic patients undergoing coronary stenting. In the present study, there were non significant changes in plaque progression and lumen loss as well as in vessel remodeling at the peristent reference segments from poststenting to follow-up between patients receiving and not receiving simvastatin treatment. Our study suggests that simvastatin in moderate doses does not inhibit plaque progression and lumen narrowing at stent edges in nor-mocholesterolemic patients who have undergone BMS implantation.
This study has several limitations that require a mention. First, being a retrospective study, it is subjected to the limitations inherent in this type of clinical investigation. Second, this single-center study included only a small number of patients. Third, we did not assess the changes in EEM, lumen, and plaque areas at sites that were further distant from the stent edges, i.e, segments that were not affected by the stent or balloon. Fourth, the follow-up LDL cholesterol level was 94±34 mg/dL in the simvastatin group. This level might not be high enough to affect the change in the plaque volume. Fifth, we did not compare the effects of low-dose statin with moderate or high-dose statin therapy on plaque regression and vascular remodeling. Therefore, further prospective, randomized, large-scale studies are required. We conclude that the conventional dose of simvastatin does not inhibit plaque progression and lumen loss in the peristent reference segments of normocholesterolemic patients who have undergone BMS implantation.
Figures and Tables
Fig. 1
Serial area changes (follow-up value minus poststenting value) in the mean external elastic membrane (EEM), lumen, and plaque and media (P&M) areas based on the simvastatin therapy at the proximal (A) and distal (B) peristent reference segments.

Acknowledgments
This study was supported by grants of the Korea Health 21 R&D Project Ministry of Health & Welfare, Republic of Korea (A050174), and Cardiovascular Research Foundation Asia.
References
1. Hoffmann R, Mintz GS, Popma JJ, et al. Chronic arterial responses to stent implantation: a serial intravascular ultrasound analysis of Palmaz-Schatz stents in native coronary arteries. J Am Coll Cardiol. 1996. 28:1134–1139.
2. Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996. 94:1247–1254.
3. Mudra H, Regar E, Klauss V, et al. Serial follow-up after optimized ultrasound-guided deployment of Palmaz-Schatz stents: in-stent neointimal proliferation without significant reference segment response. Circulation. 1997. 95:363–370.
4. Weissman NJ, Wilensky RL, Tanguay JF, et al. Extent and distribution of in-stent intimal hyperplasia and edge effect in a non-radiation stent population. Am J Cardiol. 2001. 88:248–252.
5. Jimenez-Quevedo P, Sabate M, Angiolillo DJ, et al. Vascular effects of sirolimus-eluting versus bare-metal stents in diabetic patients: three-dimensional ultrasound results of the Diabetes and Sirolimus-Eluting Stent (DIABETES) Trial. J Am Coll Cardiol. 2006. 47:2172–2179.
6. Serruys PW, Degertekin M, Tanabe K, et al. Vascular responses at proximal and distal edges of paclitaxel-eluting stents: serial intravascular ultrasound analysis from the TAXUS II trial. Circulation. 2004. 109:627–633.
7. Honda Y, Grube E, de La Fuente LM, Yock PG, Stertzer SH, Fitzgerald PJ. Novel drug-delivery stent: intravascular ultrasound observations from the first human experience with the QP2-eluting polymer stent system. Circulation. 2001. 104:380–383.
8. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004. 350:1495–1504.
9. Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004. 291:1071–1080.
10. Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003. 91(4A):4B–8B.
11. Corsini A, Raiteri M, Soma MR, Gabbiani G, Paoletti R. Simvastatin but not pravastatin has a direct inhibitory effect on rat and human myocyte proliferation. Clin Biochem. 1992. 25:399–400.
12. Roberts WL, Moulton L, Law TC, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications: part 2. Clin Chem. 2001. 47:418–425.
13. Walter DH, Schachinger V, Elsner M, Dimmeler S, Zeiher AM. Platelet glycoprotein IIIa polymorphisms and risk of coronary stent thrombosis. Lancet. 1997. 350:1217–1219.
14. Strandberg TE, Vanhanen H, Tikkanen MJ. Effect of statins on C-reactive protein in patients with coronary artery disease. Lancet. 1999. 353:118–119.
15. Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998. 98:839–844.
16. Park SY, Kwak JJ, Park SH. Dose dependent changes of lipid profiles, IL-6 and CRP in unstable angina patients after simvastatin therapy. Korean Circ J. 2003. 33:663–670.
17. Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. Circulation. 1999. 99:3227–3233.
18. Son JW, Koh KK. Effects of statins on endothelium: vasomotor eunction, inflammation, and hemostasis. Korean Circ J. 1999. 29:1016–1031.
19. Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease: correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995. 92:3172–3177.
20. The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994. 344:1383–1389.
21. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996. 335:1001–1009.
22. Hong YJ, Jeong MH, Lim JH, et al. The prognostic significance of statin therapy according to the level of C-reactive protein in acute myocardial infarction patients who underwent percutaneous coronary intervention. Korean Circ J. 2003. 33:891–900.
23. Nakagawa T, Kobayashi T, Awata N, et al. Randomized, controlled trial of secondary prevention of coronary sclerosis in normocholesterolemic patients using pravastatin: final 5-year angiographic follow-up of the Prevention of Coronary Sclerosis (PCS) study. Int J Cardiol. 2004. 97:107–114.
24. Tamura A, Mikuriya Y, Nasu M. Effect of pravastatin (10 mg/day) on progression of coronary atherosclerosis in patients with serum total cholesterol levels from 160 to 220 mg/dL and angiographically documented coronary artery disease. Am J Cardiol. 1997. 79:893–896.
25. Suzuki M, Saito M, Nagai T, Saeki H, Kazatani Y. Prevention of positive coronary artery remodeling with statin therapy in patients with coronary artery diseases. Angiology. 2006. 57:259–265.
26. Jensen LO, Thayssen P, Mintz GS, Carlier SG, Pedersen KE, Haghfelt T. Effect of simvastatin on coronary lesion site remodeling. Cardiology. 2006. 106:256–263.
27. Petronio AS, Amoroso G, Limbruno U, et al. Simvastatin does not inhibit intimal hyperplasia and restenosis but promotes plaque regression in normocholesterolemic patients undergoing coronary stenting: a randomized study with intravascular ultrasound. Am Heart J. 2005. 149:520–526.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download