Abstract
Background and Objectives
Vascular endothelial growth factor (VEGF) is a potent endothelial cell-specific mitogen. This study was undertaken to test the hypothesis that the neointima hyperplasia induced by a balloon injury is inhibited by blocking VEGF and VEGF receptor-1 (VEGFR-1) with anti-VEGF peptides.
Materials and Methods
Anti-VEGF RRKRRR peptide (dRK6) and anti-VEGFR-1 peptide (anti-flt-1) were synthesized at Pohang University of Science and Technology, Korea. Male Sprague-Dawley rats, weighing 300-350 g, were subcutaneously injected 0.5 mg/kg of dRK6 or 0.5 mg/kg of anti-flt-1, dissolved in phosphate buffer solution, 2 days before induction of a carotid balloon-injury, and then daily in the same manner post carotid balloon injury for 2 weeks.
Results
Neointima formation was suppressed in both the dRK6 and anti-flt-1 groups compared to that in the untreated controls at 2 weeks post carotid balloon-injury (neointimal area; control group 0.44±0.09 mm2, dRK6 group 0.25±0.05 mm2, anti-flt-1 group 0.19±0.05 mm2, p<0.01). Anti-flt-1 peptide and dRK6 reduced the numbers of proliferative bromodeoxyuridine-labeled cells in the neointima (control group 16.4±10.6%, dRK6 group 3.7±2.1%, anti-flt-1 group 5.9±3.4%, p<0.05). In addition, an inflammatory response, as determined by monocyte chemoattractant protein-1 and interleukin-6 upregulation, which was evident in the controls, was inhibited by both dRK6 and anti-flt-1.
Restenosis after percutaneous intervention for coronary artery disease is due to the intimal tissue growth caused by the migration and proliferation of vascular smooth muscle cells (VSMCs).1)2) Brachytherapy3)4) and drug-eluting stents5)6) have been introduced to prevent this proliferation.
Vascular endothelial growth factor (VEGF) is a potent endothelial cell-specific mitogen, which enhances vascular permeability and stimulates angiogenesis.7)8) Therapeutic angiogenesis, using VEGF gene transfer, has been performed in selected patients with critical limb ischemia.9)10) Studies have also been performed with VEGF for ischemic heart disease.11-13) VEGF direct gene transfer may passivate endovascular stents by accelerating stent endothelialization. thereby reducing in-stent thrombus formation and obstruction due to intimal thickening.14)15) However, Swanson et al.16) reported that VEGF-eluting stents did not accelerate re-endothelialization or inhibit restenosis.
Recent experimental studies have demonstrated the collaterogenic effect of monocytes/macrophages, T lymphocytes, bone marrow-derived progenitor cells, VEGF family members, tumor necrosis factor-alpha and basic fibroblast growth factor. However, the Janus phenomenon implies that these cells and molecules may worsen atherosclerosis.17) VEGF may have an atheroprotective effect because it protects endothelial cells against the toxic effects of oxidized low density lipoprotein; however, by securing endothelial integrity and survival, VEGF may conversely favor plaque growth and destabilization.18)
VEGF regulates angiogenesis by primarily interacting with main two tyrosine kinase receptors, VEGF receptor-1 (VEFGR-1, also known as Fms-like tyrosine kinase, Flt1) and VEGF receptor-2 (VEGFR-2, also known as fetal liver kinase, Flk1).19) VEGFR-1 is expressed on vascular endothelial cells as well as non-endothelial cells, including smooth muscle cells, monocytes, trophoblasts, mesangial cells and osteoblasts. VEGFR-2, which has tyrosine kinase activity, is mostly located on vascular endothelial cells and is an important factor under both physiologic (wound healing, ovulation and menstruation) and pathologic conditions (tumor growth).18)
By screening peptide libraries, a soluble arginine-rich hexapeptide (e.g., RRKRRR, dRK6) and anti-flt-1 (Gly-Asn-Gln-Trp-Phe-Ile or GNQWFI) were synthesized at Pohang University of Science and Technology, Korea.20)21) These workers reported that dRK6 can bind VEGF and; thereby, block the interaction between VEGF165 and VEGF receptors as well as the growth and metastasis of VEGF-secreting HM7 human colon carcinoma cells in nude mice.20) Moreover, anti-flt-1 peptide has shown specificity for VEGFR-1 binding and inhibitions of VEGF, placental growth factor (PlGF) and VEGF/PlGF heterodimer binding to VEGFR-1.21)
Shibata et al.22) have shown that VEGF is involved in the process of restenosis, due to its angiogenic properties, and induction of myocyte chemotaxis in pigs following stenting. However, it is not clearly known whether the above-mentioned anti-VEGF peptides, dRK6 and anti-flt-1, suppress neointimal formation following percutaneous intervention. Therefore, whether the neointima formation is inhibited by dRK6 (a VEGF antipeptide) or anti-flt-1 (a VEGFR-1 antipeptide) in balloon-injured rat carotid arteries was investigated.
The dRK6 and anti-flt-1 were synthesized at Pohang University of Science and Technology, Korea, as described previously.20)
Male Sprague-Dawley rats, weighing 300-350 g, were used in this experiment, and were subcutaneously injected with 0.5 mg/kg of dRK6 (N=5) or 0.5 mg/kg of anti-flt-1 (N=5), which were dissolved in phosphate buffer solution (PBS), 2 days prior to induction of a carotid balloon-injury, and then daily for 2 weeks post carotid balloon injury. Control animals received PBS alone (N=5) or the reverse peptide of dRK6 (N=4), again daily for 2 weeks post carotid injury.
Arterial injury was induced using the balloon denudation technique.23) After induction of anesthesia with an injection of xylazine (4.6 mg/kg) and ketamine (70 mg/kg), a cervical midline incision was made, with the bifurcation of the left carotid artery then exposed. Blood flow to the site of the surgical manipulation was temporarily interrupted by lifting the left common, internal and external carotid arteries with the use of surgical ligatures. A 2 Fr balloon catheter (Fogarty, Baxter, USA) was then introduced via an arteriotomy in the external carotid artery and advanced to the proximal edge of the omohyoid muscle. To produce denudation and a mechanical stretching injury of the left common carotid artery, the balloon was inflated to generate slight resistance, and then withdrawn from the proximal part of the omohyoid to the carotid bifurcation three times. Following this procedure, the catheter was removed and the insertion ligated.
Two weeks after induction of the balloon injury, animals were euthanized, and the left carotid and the left femoral arteries exposed. Via a puncture in the left femoral artery, a catheter was introduced into the ascending aorta, with the aorta fixed with 4% formaldehyde at 90-100 mmHg for 5 minutes. Following the initiation of perfusion and fixation, animals were euthanized with an overdose of thiopental sodium via a tail vein. Left carotid arteries were stored in 4% formaldehyde for 24 hours, and then embedded in paraffin for sectioning at thicknesses of 5 µm. Hematoxylin-eosin staining, elastin fiber staining and immunochemical studies were then conducted.
After recording digital images at ×100 magnification, the luminal (the area circumscribed by the intimal border), intimal (the area between the lumen and the internal elastic lamina) and the medial (the area between the internal and external elastic lamina) areas were delineated by computerized morphometry (AxioVsion LE v 4.1.1, Carl Zeiss Vision GmbH, Germany).
To detect proliferative cells, bromodeoxyuridine (BrdU) (Sigma-Aldrich Co, St. Louis, MO, USA) was injected intramuscularly 2 hours (100 mg/kg) before euthanasia. Immunohistochemistry was performed using a monoclonal antibody (Dako Cytomation California Inc., Carpinteria, CA, USA) against BrdU. BrdU-labeled cells in the intima were counted at ×400 magnification. BrdU-labeling indices (the fraction of labeled nuclei times 100) were calculated in the intima to assess the rate of cell replication. In addition, immunohistochemistry was performed using antibodies against VEGF (A-20: sc152, Santa Cruz Biotechnology, Santa Cruz, CA, USA), VEGFR-1 (Flt-1, Lab Vision, Fremont, CA, USA), VEGFR2 (Flk-1/DR/VEGFR2, Lab Vision), monocyte chemoattractant protein-1 (MCP-1, anti-mouse CCL2/MCP-1 antibody, R&D Systems, Minneapolis, MN, USA) and IL-6 (anti-rat IL-6 antibody, R&D Systems). Reaction products were developed with diaminobenzidine (Sigma-Aldrich Co) and counterstained with hematoxylin.
The carotid arteries in the control group developed significant neointimal formation by day 14 (Fig. 1), where those in the dRK6 and anti-flt-1 groups showed less neointimal formation (neointimal area; control group 0.44±0.09 mm2, dRK6 group 0.25±0.05 mm2 and anti-flt-1 group 0.19±0.05 mm2, p<0.05) (Fig. 1, 2) (Table 1).
The localizations of VEGF and VEGFR-1 were immunohistochemically studied in rats. VEGF and VEGFR-1 were immunopositive in the nuclei of neointimal cells in the control group on day 14 (Fig. 4). The administration of the anti-flt-1 peptide showed a slight effect on the expression of VEGF, and both dRK6 and anti-flt-1 reduced the VEGFR-1 immunoreactivities. VEGFR-2 was expressed on the luminal side of the control group, but was absent in the dRK6 and anti-flt-1 groups (Fig. 4).
Immunohistochemical staining performed 14 days after balloon injury showed increased monocyte chemoattractant protein-1 (MCP-1) and IL-6 immunoreactivities in neointimal cells, which were attenuated by dRK6 and anti-flt-1 (Fig. 5).
In contrast to VEGF and its receptor, VEGFR-2, PlGF and its receptor, VEGFR-1, have been poorly investigated and largely neglected.18) However, recent gene-targeting studies have indicated that PlGF and VEGFR-1 are key regulators of the angiogenic switch under pathological conditions.18)24) The anti-VEGFR-1monoclonal antibody (mAb) has been reported to block neovascularization in the ischemic retina18)25) and to dose-dependently block angiogenesis and growth of human epidermoid A431 tumors in nude mice.26) Autiero et al.18) used anti-VEGFR-1 and anti-VEGFR-2 mAb to study their effects on the growth and stability of initial (avascular) fatty streak lesions as well as interme-diate and advanced (vascularized) plaques in atherosclero-sis-prone apolipoproteinE-deficient mice. Treatment with anti-VEGFR-1 mAb was found to reduce the sizes of the early and intermediate lesions as well as the growth of advanced atherosclerotic lesions. However, anti-VE-GFR-2 mAb failed to affect atherosclerotic plaque deve-lopment at any stage. However, surprisingly, neither anti-VEGFR-1 mAb nor anti-VEGFR-2 mAb blocked angiogenesis in atherosclerotic lesions or the surrounding adventitia. Therefore, it appears anti-VEGFR-1 mAb suppressed plaque growth and vulnerability by inhibiting inflammatory cell infiltration, independent of angiogenesis; whereas, anti-VEGFR-2 mAb, which normally blocks angiogenesis, was ineffective. It is also important to note that the inhibition of inflammatory cell infiltration is related to the suppression of plaque growth.
In this study, anti-peptides, instead of monoclonal antibodies, were used to block either VEGF or VEGFR-1 in the balloon-injured rat carotid artery. Yoo et al.27) demonstrated that dRK6 suppressed ongoing paw inflammation in collagen-induced arthritis and blocked the VEGF-induced production of proinflammatory cytokines in a mouse model. Moreover, the anti-flt-1 peptide (Gly-Asn-Gln-Trp-Phe-Ile or GNQWFI), which inhibits VEGFR-1 binding, was also identified by screening a synthetic peptide library.21) The anti-flt-1 peptide was found to bind specifically with VEGFR-1, but inhibit the bindings of VEGF, PlGF and the VEGF/PlGF heterodimer to VEGFR-1. Moreover, anti-flt-1 peptide effectively blocked the VEGF-induced migration of endothelial cells and their ability to form capillarylike structures on a fibrin gel-based in vitro angiogenesis system.21) Furthermore, the growth and metastasis of VEGF-secreting tumor cells were also significantly inhibited by subcutaneous injections of anti-flt-1 peptide in nude mice.21) The blocking of either VEGF or VEGFR-1 by anti-peptides has some advantages over gene transfer or the use of a monoclonal antibody; it does not use a viral vector, does not cause an antigen-antibody reaction and is less expensive. The present study has demonstrated that both the blocking of VEGF by dRK6 and VEGFR-1 by anti-flt-1 peptide reduces neointimal formation in a balloon-injured rat artery. To our knowledge, this is the first study to evaluate the effect of dRK6 or anti-flt-1 peptide on neointima formation following induction of a balloon-injury.
Ohtani et al.28) reported that the increased expression and activity of VEGF are essential for the development of experimental restenosis following an intraluminal injury, due to the recruitment of monocyte-lineage cells; and that blockade of VEGF by soluble VEGF receptor 1 gene transfer attenuated neointimal formation following an intraluminal injury and inhibited the increased expression of inflammatory factors, such as monocyte chemoattractant protein-1 (MCP-1) and VEGF in rabbits, rats and mice. Yamada et al.29) showed that VEGF-mediated angiogenesis and inflammation are actually mediated by monocyte chemoattractant protein-1 (MCP-1). This present study also showed that MCP-1 was reduced in the dRK6 and anti-flt-1 peptide groups, which supports the role of MCP-1 in neointimal formation following vascular injury and the suppression of neointimal formation due to dRK6 and anti-flt-1 peptide.
De Leon et al.30) reported a BrDU labeling index of 27.5±3.8% in balloon injured rat carotid arteries on day 3 post-injury. In the present study, BrDU positive cells in the neointima were reduced in the dRK6 and anti-flt-1 peptide groups compared to the control group on day 14 post-injury, suggesting VEGF anti-peptides suppressed neointimal proliferating cells post-injury.
There was no difference between anti-flt-1 peptide and dRK6 with respect to reduced neointimal formation, but this will require further elucidation. The re-endothelialization in a rat carotid artery following a balloon injury was not conducted in this study, but this might also be affected by VEGF inhibition. Swanson et al.16) reported that a VEGF-eluting stent did not accelerate re-endothelialization or inhibit restenosis, but appeared to reduce stent thrombosis. Theoretically, inhibition of VEGFR-1 by anti-flt-1 peptide might be more beneficial than the blocking of VEGF itself by dRK6, as this does not affect VEGFR-2 mediated endothelialization. In the present study, the dRK6 group tended to show an increased incidence of thrombosis (data not shown), which may have been due to the suppression of re-endothelialization by dRK6. Studies on the side effects of anti-flt-1 or dRK6 on the possible thrombogenic potential, as well as their effects on angiogenesis and re-endothelialization are still required. Also, the immunoreactivities of MCP-1 and IL-6 were not quantitatively analyzed in this study.
Many studies have been performed on the effects of blocking VEGF or VEGFR-1, using anti-peptides, in cancer and rheumatic disease, but no study has been conducted on the use of anti-peptides to reduce restenosis. Therefore, anti-VEGF peptides were concluded to potentially offer a route for the development of therapeutic agents that will prevent restenosis following percutaneous coronary intervention.
Figures and Tables
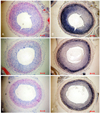 | Fig. 1Representative carotid arteries from the control (A and B), dRK6 (C and D) and anti-flt-1 groups (E and F) 14 days after balloon injury illustrating hematoxylin and eosin staining (A, C and E) and elastic fiber staining (B, D and F). dRK6 and anti-flt-1 both inhibited neointima formation 14 days post-injury. Magnification ×100. Bar indicates 100 µm. dRK6: anti-vascular endothelial growth factor RRKRRR peptide. RRKRRR: Arg-Arg-Lys-Arg-Arg-Arg. |
 | Fig. 2Neointimal area on day 14 after balloon injury. Neointimal areas were reduced in the dRK6 (n=5) and anti-flt-1 groups (n=5) vs. the control group (n=5). *means p<0.05, †p<0.01 by Bonferroni's test. dRK6: anti-vascular endothelial growth factor RRKRRR peptide. RRKRRR: Arg-Arg-Lys-Arg-Arg-Arg. |
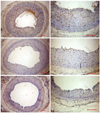 | Fig. 3BrDU staining of balloon-injured rat carotid arteries of the control (A and B), dRK6 (C and D) and anti-flt-1 groups (E and F) 14 days after balloon injury. Both dRK6 and anti-flt-1 reduced the numbers of BrDU positive cells, reflecting reduced neointimal cell proliferation 14 days post-injury. Magnification ×100 (A, C and E) and ×200 (B, D and F). Bar indicates 100 µm. BrDU: bromodeoxyuridine, dRK6: anti-vascular endothelial growth factor RRKRRR peptide, RRKRRR: Arg-Arg-Lys-Arg-Arg-Arg. |
 | Fig. 4Arterial cross sections taken 14 days post-injury were immunohistochemically stained for VEGF, VEGFR-1 (flt-1) and VEGFR-2 (flk-1). Strong VEGF and VEGFR-1 immunoreactivities in the media of the control group. dRK6 or anti-flt-1 treatments reduced VEGFR-1 immunoreactivities on day 14, but no changes were observed in the VEGF immunoreactivities. VEGFR-2 was expressed on the luminal side of the control group, but was absent in the dRK6 andanti-flt-1 groups. Magnification ×400. # indicates lumen. Bar indicates 100 µm. VEGF: vascular endothelial growth factor, VEGFR-1: VEGF receptor-1, VEGFR-2: VEGF receptor-2, BrDU: bromodeoxyuridine, dRK6: anti-VEGF RRKRRR peptide, RRKRRR: Arg-Arg-Lys-Arg-Arg-Arg. |
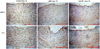 | Fig. 5Carotid artery sections from the control, dRK6 and anti-flt1 groups 14 days post-injury stained with MCP-1 and IL-6. The immunoreactive MCP-1 and IL-6 in neointimal cells of the control group were attenuated by the administration of both dRK6 and anti-flt-1 peptides. Magnification ×400. Bar indicates 50 µm. dRK6: anti-vascular endothelial growth factor RRKRRR peptide, MCP-1: monocyte chemoattractive protein-1, IL-6: Interleukin-6, RRKRRR: Arg-Arg-Lys-Arg-Arg-Arg. |
Acknowledgments
This study was supported by the operating grant, SanHak-2004-12, from The Korean Society of Cardiology, and was orally presented at the American College of Cardiology on Mar 27th, 2007.
References
1. Post MJ, Borst C, Pasterkamp G, Haudenschild CC. Arterial remodeling in atherosclerosis and restenosis: a vague concept of a distinct phenomenon. Atherosclerosis. 1995. 118:S115–S123.
2. Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996. 94:1247–1254.
3. Teirstein PS, Massullo V, Jani S, et al. Three-year clinical and angiographic follow-up after intracoronary radiation: results of a randomized clinical trial. Circulation. 2000. 101:360–365.
4. Kim HS, Yoon MH, Oh YT, et al. The effect of external beam radiation on neointimal formation in the rat carotid injury model. Korean Circ J. 1998. 28:173–182.
5. Sousa JE, Costa MA, Abizaid A, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001. 103:192–195.
6. Cho MC, Kwak NJ, Piao H, et al. Effect of paclitaxel local delivery on neointimal formation after endothelial denudation of the rat carotid artery. Korean Circ J. 2000. 30:198–207.
7. Klagsbrun M, D'Amore PA. Regulators of angiogenesis. Annu Rev Physiol. 1991. 53:217–239.
8. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995. 146:1029–1039.
9. Isner JM, Pieczek A, Schainfeld R, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996. 348:370–374.
10. Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998. 97:1114–1123.
11. Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998. 98:2800–2804.
12. Schumacher B, von Specht BU, Haberstroh J, Pecher P. The stimulation of neo-angiogenesis in the ischemic heart by the human growth factor FGF. J Cardiovasc Surg. 1998. 39:445–453.
13. Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999. 100:468–474.
14. Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation. 2003. 107:2677–2683.
15. Walter DH, Cejna M, Diaz-Sandoval L, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004. 110:36–45.
16. Swanson N, Hogrefe K, Javed Q, Malik N, Gershlick AH. Vascular endothelial growth factor (VEGF)-eluting stents: in vivo effects on thrombosis, endothelialization and intimal hyperplasia. J Invasive Cardiol. 2003. 15:688–692.
17. Epstein SE, Stabile E, Kinnaird T, Lee CW, Clavijo L, Burnett MS. Janus phenomenon: the interrelated tradeoffs inherent in therapies designed to enhance collateral formation and those designed to inhibit atherogenesis. Circulation. 2004. 109:2826–2831.
18. Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003. 1:1356–1370.
19. Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001. 26:25–35.
20. Bae DG, Gho YS, Yoon WH, Chae CB. Arginine-rich anti-vascular endothelial growth factor peptides inhibit tumor growth and metastasis by blocking angiogenesis. J Biol Chem. 2000. 275:13588–13596.
21. Bae DG, Kim TD, Li G, Yoon WH, Chae CB. Anti-flt1 peptide, a vascular endothelial growth factor receptor 1-specific hexapeptide, inhibits tumor growth and metastasis. Clin Cancer Res. 2005. 11:2651–2661.
22. Shibata M, Suzuki H, Nakatani M, et al. The involvement of vascular endothelial growth factor and flt-1 in the process of neointimal proliferation in pig coronary arteries following stent implantation. Histochem Cell Biol. 2001. 116:471–481.
23. Moon KW, Lee JM, Chang KU, et al. Oral everolimus reduces adventitial cell activation and neointima formation in balloon-injured rat carotid artery. Korean Circ J. 2004. 34:983–991.
24. Luttun A, Tjwa M, Carmeliet P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): novel therapeutic targets for angiogenic disorders. Ann N Y Acad Sci. 2002. 979:80–93.
25. Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001. 7:575–583.
26. Stefanik DF, Fellows WK, Rizkalla LR, et al. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol. 2001. 55:91–100.
27. Yoo SA, Bae DG, Ryoo JW, et al. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-alpha and IL-6 by human monocytes. J Immunol. 2005. 174:5846–5855.
28. Ohtani K, Egashira K, Hiasa K, et al. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004. 110:2444–2452.
29. Yamada M, Kim S, Egashira K, et al. Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2003. 23:1996–2001.
30. De Leon H, Ollerenshaw JD, Griendling KK, Wilcox JN. Adventitial cells do not contribute to neointimal mass after balloon angioplasty of the rat common carotid artery. Circulation. 2001. 104:1591–1593.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download