Abstract
Endovascular aneurysm repair (EVAR) was initially introduced as a less invasive alternative to conventional open repair. EVAR was subsequently adopted as a treatment option for abdominal aortic aneurysm. In Korea, open repair is more widely available than EVAR, although EVAR can be performed in several hospitals. Due to the rapidly aging population in Korea, there has been a shift from private healthcare to government-regulated universal coverage and EVAR may be a more feasible option for cardiovascular interventionalists in these days. The improvement of EVAR was rapidly attained by many pioneers for the last two decades. Although issues such as indications and durability of EVAR remain to be elucidated, its application can be extended further of milder invasiveness-related effects on comorbidities and less discomfort to patients. Aortic stent-grafting has been performed for various aortoiliac pathologies over the last 13 years at our cardiovascular center.1-3) This article presents a comprehensive review on EVAR by focusing on the clinical trials, indications, complications, and expertise in decision making for EVAR.
Endovascular abdominal aortic aneurysm (AAA) repair was initially introduced as a less invasive alternative to conventional open repair-Parodi in 1990,4) and was adopted as one of the treatment options for AAA. In the past, endovascular aneurysm repair (EVAR) was accepted as a compatible method to more conventional method from the viewpoint of its safety and favorable outcomes.5-7) Earlier clinical studies on EVAR have reported significant decrease in intensive care unit (ICU) stay, hospital stay, major complications such as bleeding, and the 30-day mortality. Furthermore, due to its advantages including prompt recovery and early ambulation, EVAR had emerged as a substitute to surgery particularly for treating elderly patients with multiple comorbidities. However, some conflicting results have been reported from more recent studies that assessed the mid to long-term impacts of EVAR.
For instance, some studies8)9) revealed inconsistencies in the early positive outcomes of EVAR. While one study reported three to six years of sustained benefits, another study raised concerns regarding its accepted durability. In addition, other studies10-14) discussed concerns regarding the increased risks of possible graft failure, that would necessitate re-intervention or surgical conversion in the later phase. Endovascular Aneurysm Repair (EVAR) trial 1, that included patients who could endure both surgery and EVAR, reported patients significantly lower 30-day mortality rates, but an increase of post-operative re-intervention rates with EVAR.15)
In EVAR trial 2, researchers compared patients who received EVAR with only medically treated patients without intervention, and found that the former group had significantly higher rates of all-cause and aneurysm-related mortality, and EVAR was reported to be less cost-effective. Therefore, the researchers concluded that careful case selection and clinical follow-ups are critical in EVAR.16) In the Dutch Randomized Endovascular Aneurysm Management (DREAM) trial, open repair group and endovascular repair group were compared based on the accumulated aneurysm-related mortality rates. The results obtained did not reveal any significant differences in the primary end-points. Therefore, short-term advantages such as immediate post-interventional safety and early positive outcomes should neither be considered the best determinants of prognosis of endovascular repair, nor be related to long-term outcomes.17)18) However, there have not been any randomized prospective clinical trials on the long-term outcomes in patients with a high operative risk.
In Korea, open repair is more widely available than EVAR, although EVAR can be performed in several hospitals. The approved devices in Korea are Zenith (Cook Medical, Bloomington, IN), Excluder (Gore & Associates, Flagstaff, AZ), and AneuRx (Medtronic, Inc., Santa Rosa, CA). In the past 2 years, only 138 EVARs have been performed in Korea. Zenith was used for 24 procedures in 2005 and 84 procedures in 2006; Excluder was used for 13 procedures in 2005 and 8 procedures in 2006; the remaining 9 procedures were performed using SEAL (S&G biotech, Sungnam, Gyeonggi-do, Korea), a domestic device that has been recently approved for use in Korea. Due to the rapidly aging population in Korea, there has been a shift from private healthcare to government-regulated universal coverage. Korean government insurance bears for 90% expense of the EVAR device and the patient bears the remaining 10%.
The mortality rate of ruptured AAA is more than 90%, however, it can be decreased by the emergent implantation of stent-graft.19) Hence, it is crucial to manage AAA prior to its rupture, although the possibility and timing of rupture may vary considerably for each case. Generally, the risk of aneurysmal rupture increases substantially if the diameter of AAA ranges between 5 to 6 cm. For example, the annual risk of AAA rupture is 5-11%, when the diameter of AAA is more than 5 cm.20) The following factors impose a high risk for AAA rupture: AAA diameter or annual progression of more than 0.6 cm, heavy smoking, family history of vascular diseases, poorly controlled hypertension, eccentric shape of the aneurysm, and female gender.21)
In our experiments with patients, mortality rates could not be reduced considerably by performing emergency procedures after AAA rupture; this was due to progressive multi-organ failures including renal shut-down. Therefore, it is critical to detect the progression of AAA during the early stage with close clinical and radiographic follow-ups. Fig. 1 shows a successful endovascular stent-graft repair in a patient with AAA which was impending rupture. Fig. 2 shows an urgent stent-graft repair in a patient with aortic rupture who was diagnosed with Bechet disease.
From our experiences, accurate pre-procedural analysis using imaging techniques and the selection of the most appropriate instruments are very effective for the successful management of the cases of AAA and biiliac artery with vascular tortuosity. More importantly, the level of experience and expertise of the surgeons were found to be critical for successful implantations of stent-grafts.
Following procedures and instruments were used for cases that were previously considered as contraindications for EVAR (acute-angled aneurysm extremely extended to the renal or biiliac artery): incorporation of an uncovered stent for facilitating adequate stent-graft apposition, the staged embolization of the internal iliac artery, and vertical modification of the puncture site. With these novel techniques, the number of successful cases of EVAR has increased gradually when compared to that of conventional open repair. Considering our past data, a relatively acceptable indication of stent-grafting for AAA is an aneurysmal diameter ranging from 5 to 5.5 cm, depending on the patient's native vessel diameter or the rate of aneurysm enlargement (1 cm/year).
New onset or aggravation of pain and other medically intractable conditions such as rupture with either acute or chronic presentation despite adequate medical treatments, are indications for EVAR.22)23) However, with considerable variations in every aortic aneurysm, it is not easy to clearly state the appropriateness of EVAR, particularly white treating patients with grave comorbidities, even in large, randomized clinical trials.
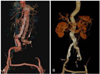
Manifested complications such as migration of stent-graft, spontaneous endoleak (Fig. 3) or porosity, and stent thrombosis (Fig. 4) have been detected during followups, leading to either re-intervention or operative correction in some cases.24) The current classification system for endoleaks and endotension is detailed in Table 1.25) Type I or III endoleak should be corrected because the aneurysmal sac in these cases would be exposed to systemic blood pressure, leaving no possibility for spontaneous resolution.25)26) Therefore, in the absence of a sound evidence of initial endoleak, it is critical to detect the existence of endoleak by using computed tomography follow-up. Moreover it is helpful to pursue additional interventions such as coiling or redo stent-graft implantation to prevent the increase in pressure overload to the aneurysm.
In general, cases that required additional procedures were considered as clinically unsuccessful. However, broadening the implication of success and by considering subsequent procedures important, the impact of EVAR on the effective prognosis of patients would be substantial. In addition, it is important to manage comorbidities intensively with antihypertensive, antilipidemic, and antiplatelet agents for better patients' prognosis and to consider stent durability and patency against the remodeling of aorta or thrombosis.
The research and development of EVAR has gained considerable attention and speed over the past decades due to the substantial effort from the pioneers. Although some difficult questions remain to be answered, such as the correct indications for and durability of EVAR, this intervention does have advantages such as less invasiveness-related effects on comorbidities and greater comfort to patients.
Figures and Tables
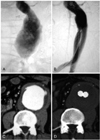 | Fig. 1Successful endovascular aneurysmal repair in a patient with abdominal aortic aneurysm (AAA). Pre-interventional (A) and post-stent-graft implantation (B) aortograms show marked improvement in distal blood flow propagation. Pre-interventional (C) and post-stent-graft implantation (D) computer tomography show stabilization of the aortic diameter and extraluminal thrombosis without leakage. |
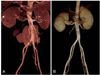 | Fig. 2An urgent stent-graft repair in a patient with abdominal aortic rupture who was diagnosed with Beçhet disease. Pre-interventional computer tomography (A) shows leakage around the lower abdominal aorta, and the post-interventional image (B) shows no further deterioration or complication. |
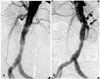 | Fig. 3Type I endoleak after stent-graft implantation. A: immediate post stent-graft implantation aortogram shows no visible endoleak. B: one year after stent-graft implantation, there occurred type I endoleak (black arrow) with newly developed angulation of proximal stent-graft edge due to remodeling of the abdominal aorta. |
References
1. Kim BK, Park S, Ko YK, et al. Immediate and mid-term outcomes of the endovascular stent-graft treatement of abdominal aortic aneurysm. Korean Circ J. 2005. 35:583–590.
2. Shim DK, Yoon YS, Chang BC, Lee DY, Shim WH. A case of complete resolution of aortc dissection in the descending thoracic aorta treated with endovascular stent-graft implantation. Korean Circ J. 2000. 30:1583–1588.
3. Kang WC, Ko YG, Koo BK, et al. Favorable outcome of endovascular stent-graft implantation for stanford type B aortic dissection. Korean Circ J. 2003. 33:457–464.
4. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991. 5:491–499.
5. Matsumura JS, Brewster DC, Makaroun MS, Naftel DC. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 2003. 37:262–271.
6. Zarins CK, White RA, Schwarten D, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg. 1999. 29:292–305.
7. Brewster DC, Geller SC, Kaufman JA, et al. Initial experience with endovascular aneurysm repair: comparison of early results with outcome of conventional open repair. J Vasc Surg. 1998. 27:992–1003.
8. Blum U, Voshage G, Lammer J, et al. Endoluminal stent-grafts for infrarenal abdominal aortic aneurysms. N Engl J Med. 1997. 336:13–20.
9. Cuypers P, Buth J, Harris PL, Gevers E, Lahey R. Realistic expectations for patients with stent-graft treatment of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1999. 17:507–516.
10. Beebe HG, Cronenwett JL, Katzen BT, Brewster DC, Green RM. Results of an aortic endograft trial: impact of device failure beyond 12 months. J Vasc Surg. 2001. 33:S55–S63.
11. Holzenbein TJ, Kretschmer G, Thurnher S, et al. Midterm durability of abdominal aortic aneurysm endograft repair: a word of caution. J Vasc Surg. 2001. 33:S46–S54.
12. Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms. J Vasc Surg. 2000. 32:739–749.
13. Bernhard VM, Mitchell RS, Matsumura JS, et al. Ruptured abdominal aortic aneurysm after endovascular repair. J Vasc Surg. 2002. 35:1155–1162.
14. Gorham TJ, Taylor J, Raptis S. Endovascular treatment of abdominal aortic aneurysm. Br J Surg. 2004. 91:815–827.
15. Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004. 364:843–848.
16. EVAR Trial Participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005. 365:2187–2192.
17. Prinssen M, Buskens E, Blankensteijn JD. The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial: background, design and methods. J Cardiovasc Surg. 2002. 43:379–384.
18. Blankensteijn JD, de Jong SE, Prinssen M, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005. 352:2398–2405.
19. Katzen BT, Dake MD, MacLean AA, Wang DS. Endovascular repair of abdominal and thoracic aortic aneurysms. Circulation. 2005. 112:1663–1675.
20. Nevitt MP, Ballard DJ, Hallett JW Jr. Prognosis of abdominal aortic aneurysms: a population-based study. N Engl J Med. 1989. 321:1009–1014.
21. Brewster DC, Cronenwett JL, Hallett JW Jr, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms: report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003. 37:1106–1117.
22. Powell JT, Greenhalgh RM. Clinical practice: small abdominal aortic aneurysms. N Engl J Med. 2003. 348:1895–1901.
23. Scott RA, Tisi PV, Ashton HA, Allen DR. Abdominal aortic aneurysm rupture rates: A 7-year follow-up of the entire abdominal aortic aneurysm population detected by screening. J Vasc Surg. 1998. 28:124–128.
24. EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005. 365:2179–2186.
25. Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002. 35:1029–1035.
26. White GH, May J, Waugh RC, Yu W. Type I and type II endoleaks: a more useful classification for reporting results of endoluminal AAA repair. J Endovasc Surg. 1998. 5:189–191.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download