Abstract
Background and Objectives
Rupture-prone plaque, characterized by a large necrotic core, thin fibrous cap and large number of inflammatory cells, is known to be associated with acute coronary syndrome (ACS) from several autopsy and animal studies. We sought to assess in-vivo lesion characteristics of culprit lesions in patients with ACS.
Subjects and Methods
One hundred consecutive patients (mean age 60.4 years, 70 males), who underwent percutaneous coronary intervention, were analyzed for intravascular ultrasound (IVUS) radiofrequency information using IVUS-virtual histology (VH) software.
Results
Patients with ACS (n=44, mean 59.7 years, 34 males) had a lower prevalence of hypertension (45.5% vs. 67.9%, p=0.024), higher level of high-sensitivity C-reactive protein (0.36±0.36 mg/dL vs. 0.22±0.27, p=0.043), longer lesion length (22.6±8.6 mm vs. 19.3±6.9 mm, p=0.036), and more plaque rupture (63.6% vs. 10.7%, p<0.001) than those without ACS (mean 61.0 years 36 males). The lesion analysis, at a minimal luminal area, revealed that patients with ACS had a larger plaque area (12.5±5.8 mm2 vs. 10.3±4.8 mm2, p=0.043) and necrotic core (1.7±1.4 mm2 vs. 1.1±0.9 mm2, p=0.013) than those patients without ACS. Volumetric analysis over the lesion length showed that patients with ACS had larger plaque volume (9.9±4.0 mm3/mm vs. 8.3±3.4 mm3/mm, p=0.031) and necrotic core volume (1.3±1.0 mm3/mm vs. 0.8±0.6 mm3/mm, p=0.002) than those without ACS. The necrotic core volume was associated with the presence of ACS (β=0.662, p=0.041) by the IVUS-VH findings.
Figures and Tables
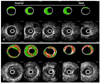 | Fig. 1Cross-sectional images of virtual histology (VH) and gray-scale intravascular ultrasound (IVUS) from distal to proximal within a same lesion in A (patient with stable angina) and B (patient with acute coronary syndrome). VH-IVUS images in patient with acute coronary syndrome (B) shows large plaque area and large amount of necrotic core area throughout the entire lesion length. |
Table 1
Clinical characteristics of the study subjects

SA: stable angina, ACS: acute coronary syndrome, FBS: fasting blood glucose, CK: creatine kinase, Tn-I: troponin-I, Hs-CRP: high sensitivity C-reactive protein, MI: myocardial infarction, STEMI: ST segment elevation myocardial infarction, LAD: left anterior desc-ending coronary artery, LCX: left circumflex coronary artery, RCA: right coronary artery, LM: left main coronary artery
References
1. Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003. 108:1664–1672.
2. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000. 20:1262–1275.
3. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995. 92:657–671.
4. Kolodgie FD, Burke AP, Farb A, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001. 16:285–292.
5. Fishbein MC, Siegel RJ. How big are coronary atherosclerotic plaques that rupture? Circulation. 1996. 94:2662–2666.
6. Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002. 106:2200–2206.
7. Bae JH, Rihal CS, Lerman A. Tissue characterization of coronary plaques using intravascular ultrasound/virtual histology. Korean Circ J. 2006. 36:553–558.
8. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS): a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001. 37:1478–1492.
9. Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006. 47:2405–2412.
10. Rodriguez-Granillo GA, McFadden EP, Valgimigli M, et al. Coronary plaque composition of nonculprit lesions, assessed by in vivo intracoronary ultrasound radio frequency data analysis, is related to clinical presentation. Am Heart J. 2006. 151:1020–1024.
11. Surmely JF, Nasu K, Fujita H, et al. Coronary plaque composition of culprit/target lesions according to the clinical presentation: a virtual histology intravascular ultrasound analysis. Eur Heart J. 2006. 27:2939–2944.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download