Introduction
The term, "aneurysm" originates from an old Greek word, "Aneurusma", meaning "widening".1) The general concept of an aneurysm is "a permanent and irreversible localized dilatation of a blood vessel".1) In a narrower meaning, the aneurysm can be defined as "a localized dilatation of 1.5 to 2.0 times the normal arterial diameter".2) The normal cut-off value for diagnosing an aneurysm is a 1.5 fold increase in the arterial diameter.3)4) In some reports, 30 mm for an infrarenal aortic aneurysm and 50 mm for a thoracic aortic aneurysm were used as absolute cut-off values.3)4) An aortic aneurysm can be defined as an "abdominal aortic aneurysm(AAA)" when it is located at the level of either a renal ostia or its distal level.1) True and false aneurysms can be classified based on the integrity of the aneurismal wall. Microscopically, all three layers(intima, media, and adventitia) of the aortic wall can be identified in a true aneurysm. However, either the adventitia only or an organized perivascular clot confines a false aneurysm.2) In a description of the aneurismal morphology, "fusiform" or "saccular" can be defined in cases when the aneurysm involves the whole or partial circumference, respectively.1)
This review describes the radiological findings, including current controversies, for diagnosis and evaluation of aortic aneurysms.
Radiological Imaging Techniques-
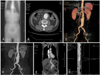
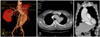
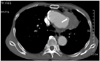
Computed tomography(CT), and especially multidetector CT(MDCT), is the most popular radiological modality for evaluating an aortic aneurysm as CT provides the best quality method for a detailed analysis of the aneurysmal morphology as well as its relationship with the adjacent arteries, such as the renal and iliac arteries.5) CT is also useful for evaluating the intrathoracic aorta due to rapid image acquisition, ECG-gating, and high spatial resolution. In addition, postprocessing techniques including volume rendering, maximum intensity projection, and multiplanar reformatting, can also provide useful information for treatment planning as well as for the lesions in and around the aneurysmal wall. In contrast to a transarterial aortogram, which is considered the gold standard in evaluating the aortic lumen, CT more clearly reveals the three-dimensional anatomy of the aorta and its branches. In addition, CT can evaluate not only the aortic lumen but also the aortic wall morphology, along with the extraaortic structures(Fig. 1).6)
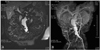
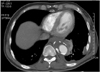
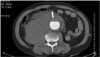
Magnetic resonance imaging(MRI) is also considered a feasible modality for evaluating an aortic aneurysm. However, in contrast to MDCT, there are some deficits such as an inferior spatial resolution, a longer examination time, more image artifacts, and higher cost. The advantages of MRI over MDCT are a lack of nephrotoxicity of the contrast media, non-enhanced scanning protocols, and tissue characterization using various pulse sequences(Fig. 2).1) In the case of an inflammatory or infected aortic aneurysm, contrast-enhanced MRI might be more sensitive for early depiction as periaortic inflammatory granulation tissue can show homogenous enhancement.7) In addition, non-enhanced cine MR angiography was introduced to overcome the long examination time of MRI, and has reduced the examination time to below 5 minutes.8)9) Although MRI is less feasible for an expedient evaluation of an aortic aneurysm compared with MDCT, the improvement in MRI techniques has been remarkable.
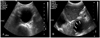
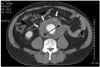
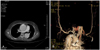
Ultrasonography(US) might be the most feasible modality for screening aortic aneurysms. US has several advantages in the diagnosis of an aortic aneurysm such as being a simple, quick, economical, and accurate modality. The reported error of US in measuring the diameter of an aneurysm was <3 mm(Fig. 3).10) An aneurysmal rupture can also be evaluated using contrast-enhanced US. Although the accuracy of depicting a rupture might be similar for US as MDCT, US may be more feasible than MDCT due to its faster performance. The ultrasonographic features of a ruptured aortic aneurysm are a delayed and longer luminal enhancement, a focal enhancement defect in the aortic wall, contrast media leakage through a mural thrombus and aortic wall, and periaortic accumulation of contrast media.11)
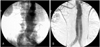
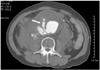
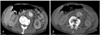
Transarterial aortography might be helpful for diagnosing an aortic aneurysm, but it is not useful for sizing an aortic aneurysm because aortography can reveal only luminal morphology without providing information on the mural thrombus or aortic wall. Usually, aortography is not used for preoperative diagnosis or planning.2) Aortography can only be used for localization and post-treatment confirmation in cases of endovascular repair(Fig. 4)1).
Image Findings
Location and morphology
An aortic aneurysm usually occurs in the infrarenal abdominal aorta with an incidence of 66-80%. The abdominal aorta is relatively less elastic and stiffer than the proximal levels, and is exposed to the highest-pressure load from reflected pressure waves from aortic bifurcation. In addition, the decreased distribution of vasa vasorum in the distal aorta may support the preference of the abdominal aorta due to its vulnerability to ischemic injury. In particular, the abdominal aortic preference is remarkably high in hypertensive subjects. Although the other levels of the aorta might be candidates for an aneurysm, the isolated involvement of the ascending aorta is not common, and is usually the result of hemodynamic stress, connective tissue disease, or an inflammatory process.2)3)12) An abdominal aortic aneurysm can extend to the iliac artery in 50% of cases.3)
An aortic aneurysm usually shows a fusiform contour, especially in cases with an atherosclerotic background. In addition, saccular and cylindroid aneurysms are not rare, especially in cases with an infection(Fig. 5).3)12) During the imaging of an aneurysm, the aneurysmal morphology, the relationship between the aorta and its branches, periaortic lesions, and the chance of rupture should be evaluated thoroughly as a basic evaluation protocol(Table 1).
Atherosclerosis
Aortic aneurysms are commonly associated with atherosclerosis(95%), which is regarded as a main causative factor(Fig. 6). Atherosclerosis in the aorta may decrease the level of aortic wall nutrition through the vasa vasorum and reduce its ability to repair the aortic wall after ischemic injury. The subsequent destruction and loss of medial collagen results in a weakening of the aortic wall strength, and the aorta becomes vulnerable to the formation of an aneurysm.2)12)
Based on a fundamental understanding of atherosclerosis, atherosclerotic plaque has a major role in vascular luminal narrowing. However, an aortic aneurysm shows expansion of the aortic lumen, even though the large part of the lumen can be occupied by a mural thrombus. Molecular medicine has been able to provide a reasonable explanation for this paradoxical property of aortic aneurysms. Since physiological inhibitors of proteases such as the tissue inhibitor of metalloproteinase II(TIMP-2) or plasminogen activator inhibitor (PAI-1) are expressed at lower levels in aortic aneurysms compared with stenotic atherosclerosis, the activity of proteases such as matrix metalloproteases(MMPs) increase and the resulting weakening of the aortic wall influences the formation of an aneurysm.13)
Intra-luminal thrombosis
An intra-luminal thrombosis might be observed as a mural thrombus within an aortic aneurysm(Fig. 1-7). The diameter of aortic aneurysm is related to its volume and the bulk of the mural thrombus as the aortic aneurysm expands in an attempt to preserve the intra-aneurysmal flow volume against the mural thrombus-induced luminal narrowing.1) It has been reported that the serological titer of plasma markers associated with fibrin formation and degradation, and the plasmin-alph2-anti-plasmin complex related to thrombus turn-over, correlates with the diameter of an abdominal aortic aneurysm.14)15) Mukaiyama et al.16) reported that radiolabeled platelets accumulated only in an abdominal aortic aneurysm, and turbulent flow was suggested to be a possible cause. Wang et al.17) reported that a hemodynamically slow flow velocity within an aortic aneurysm activates aggregation of RBC to form a rouleaux. Therefore, a mural thrombus within an aortic aneurysm might be provoked and accelerated by not only atherosclerosis but also by turbulent blood flow and activation of RBC aggregation.
The role of a mural thrombus in the progress of an aortic aneurysm has attracted considerable interest. A rupture is the most important complication of an aortic aneurysm, and is a mechanical failure of the aortic wall integrity. The tangential wall stress exceeds the tensile strength of the aneurismal wall, resulting in a rupture. If the thickness of a mural thrombus can be included in the aneurismal wall thickness, tangential stress can be decreased based on modified Laplace's law for the thin-wall model(Pressure×Diameter/Wall thickness).18) However, the influence of a mural thrombus in an aortic aneurysm is still not completely understood.
Di Martino et al.19) performed an experimental and structural static computational analysis of the biomechanics, and reported that a well-organized thrombus can decrease the pressure load of the aortic aneurismal wall. Using axisymmetric finite element analysis, Mower et al.20) also reported that an intraluminal thrombus could decrease wall stress within an abdominal aortic aneurysm. Other reports support the theory that a mural thrombus decreases aneurysmal wall stress.21)22)
In contrast, Schurink et al.18) reported that there was no significant pressure gradient present between the lumen and a thrombus that were located near the wall of an abdominal aortic aneurysm. This suggests that a mural thrombus has no role in decreasing the level of aneurysmal wall stress. Other investigators have also reported similar results.23)24)
Satta et al.25) performed a retrospective clinical study and reported that a mural thrombus paradoxically increases the risk of rupture of an abdominal aortic aneurysm. The thickness of the mural thrombus was significantly thicker in the ruptured aneurysm group than in the control group of patients. Another clinical report by Stenbaek et al.26) showed that the mural thrombus-increasing speed was higher in the ruptured aneurysm group than the control group of patients. In aneurysms where the area of the mural thrombus had increased faster than 1.5 cm2/year, 25% finally ruptured. This unexpected situation can be explained by some theories. An intraluminal mural thrombus can decrease the oxygen supply to the aneurysmal wall resulting in hypoxic wall damage with subsequent aneurysmal wall weakening and possible rupture.27) Another theory suggests that polymorphonuclear neutrophils trapped within the mural thrombus may release matrix metalloprotease-9 (MMP-9) continuously, which can subsequent weaken the aneurysmal wall, resulting in rupture.28)
Perianeurysmal inflammation and fibrosis
Chronic inflammatory processes consisting of fibrosis can be identified around the aorta in 5-23% of aortic aneurysms(Fig. 7). This process can be explained as an immune response to leaked atherosclerotic material through the aneurysmal wall.12) This situation showing an extreme end of the inflammatory process in an aortic aneurysm can be classified clinically and pathologically as a single disease entity, i.e., an inflammatory aortic aneurysm.29)30) However, both ordinary and inflammatory aortic aneurysms are regarded as a single spectrum because there is a broad borderline between them. Hayashi et al.31) observed a serial change from an ordinary uncomplicated atherosclerotic abdominal aortic aneurysm toward an inflammatory aneurysm. Clinically, this variant can lead to more severe pain, weight loss and inflammatory responses.32) Dense adhesion between the aneurysmal wall and adjacent soft tissue can occur due to exaggerated perianeurysmal inflammation and fibrosis, which can increase difficulty in surgical repair, morbidity, and mortality.7)
Contrast-enhanced CT is considered as the "diagnosis of choice" for evaluating inflammatory aortic aneurysms. Iino et al.33) reported that the sensitivity, specificity and accuracy in diagnosing inflammatory abdominal aortic aneurysm using MDCT was 83.3%, 99.7%, and 93.7%, respectively. The use of contrast-enhanced MRI is another choice for making a diagnosis. The homogenous enhancement of inflammatory tissue may be readily depicted around an aortic aneurysm.7) However, contrast-enhanced MRI is believed to be more sensitive than contrast-enhanced CT.7)34)
The major image findings consist of a thickened aneurysmal wall, perianeurysmal and retroperitoneal fibrosis, adhesions to the adjacent organs such as the duodenum, ureter, and inferior vena cava. An adhesive luminal obstruction might be hazardous to the function of these adjacent organs.7)32) In serial follow-up examinations, an inflammatory aneurysm may reveal faster expansion than an ordinary atherosclerotic aneurysm. The reported time span from an AAA to an inflammatory AAA ranges from 6.5-14 months.31)35)36)
Rupture of aneurysms
A rupture of the aneurysmal wall is the most highlighted complication of an aortic aneurysm due to the subsequent high mortality.37) The overall mortality for a ruptured abdominal aortic aneurysm has been reported to be 77-94%.3)12) An aneurysmal rupture occurs more frequently in the elderly as the rupture is closely related to the process of atherosclerosis(Fig. 6).38) An AAA rupture occurring in the posterolateral aspect may result in retroperitoneal hemorrhage. The retroperitoneal space can confine the extravasated blood, and makes up most cases transferred successfully to the emergency room. In contrast, a rupture in the anterior or anterolateral aspect may lead to an intraperitoneal hemorrhage. Although this type of rupture is less likely, most cases of sudden death are due to intraperitoneal blood loss. Clinically, sudden-onset pain is the most important symptom suggesting an aneurysmal rupture. In addition, due to the mass effect of a perianeurysmal hematoma, various symptoms and signs can occur such as obstructive jaundice, femoral neuropathy, and inguinal hernia. However, it should be noted that in most cases, there are no symptoms of an aortic aneurysm until the rupture.
An aneurysmal rupture into an adjacent organ is possible. Lower extremity swelling due to venous engorgement and high output cardiac failure can occur if the rupture causes the formation of an aortocaval fistula.9) In cases of a small rupture of the aneurysmal wall, the hematoma might be misunderstood as a unopacified bowel, enlarged lymph node, or perianeurysmal fibrosis. A chronic contained rupture showing stable hemodynamic status without evidence of blood loss may be encountered. Usually this type of rupture occurs into the retroperitoneal space. The organized hematoma may be confirmed by pathological examination, due to its chronicity.40)
Impending Rupture Signs
Sudden onset pain is the major clinical finding of an aneurysmal rupture. Usually, the pain precedes the major rupture.12) Besides pain, various image findings can be used to predict the possibility or risk of an aneurysmal rupture, such as those described below.
Size
In imaging an aneurysm, the maximal diameter is the most important factor for estimating the likelihood of an aneurysmal rupture(Fig. 8).3) In particular, the initial diameter has been reported to be more closely related to an aneurysmal rupture. If the aneurysmal diameter is<4 cm, the 6-year cumulative incidence of rupture is only 1%.39) A 4-5 cm sized aneurysm has an annual risk of rupture of 1-3%. Furthermore, a 5-7 cm and >7 cm sized aneurysms have annual risks of rupture of 6-11% and 20%, respectively.41) Of patients with aneurysms >5 cm, 25-41% were expected to experience an aneurysmal rupture within 5 years.42) In contrast to the maximal diameter, aneurysmal length was not found to be associated with the risk of rupture.43)
Expansion rate
Due to the aging process, a normal aortic diameter may increase at a speed of 0.05-0.08 mm/year.12) Nevitt et al.44) measured the average annual expansion rate of an aortic aneurysm to be 0.21 cm/year. Aortic aneurysms may expand exponentially, i.e., larger aneurysms grow faster.45) Macura et al.5) evaluated the expansion rate of abdominal aortic aneurysms with diameters of <4 cm, 4-5 cm, and >5 cm to be 2-4 mm/year, 2-5 mm/year, and 3-7 mm/year, respectively. As a sign of an impending rupture, an expansion rate "faster than 1 cm/6 months" might be a reasonable criterion.12)
Periaortic hemorrhage
Since a small amount periaortic hemorrhage may lead to a major hemorrhage, the hemorrhage itself can be an important sign of an impending rupture(Fig. 9). The anatomical continuity between the aorta and perirenal and pararenal spaces may lead to a perirenal or pararenal hematoma. The initial feature of a periaortic hemorrhage can be a finger-like or vermiform retroperitoneal hemorrhage along the perirenal fascial plane. The site of rupture of the aneurysmal wall can be observed as an indistinct hypoenhanced aortic wall due to decreased blood flow through the vasa vasorum, even though this sign has not been demonstrated to be specific for the rupture. The perianeurysmal extravasation of intravascular contrast media might be the most remarkable sign of an aneurysmal wall rupture or periaortic hemorrhage(Fig. 8).12)42)
Hyperdense crescent
At the aneurysmal wall, minute intimal tearing can lead to an intramural hematoma or hemorrhage into an adjacent mural thrombus. This pathophysiology might be depicted as an elliptic hyperdense crescent during a CT examination(Fig. 10).42)43) Histopathologically, a hemorrhage into the mural thrombus or aneurysmal wall with clefts of blood seeping from the lumen into the thrombus can be observed.46) Schwartz et al.47) reported a hyperdense crescent in 21% of ruptured and 0% in unruptured aneurysm patients. The sensitivity, specificity and positive predictive value of a hyperdense crescent in predicting a rupture has been reported to be 77%, 93%, and 53%, retrospectively.42)43)
Other impending rupture signs
In the literature, various signs of an impending rupture have been suggested in addition to the well-known signs such as a large size, rapid expansion, perianeurysmal hemorrhage, and hyperdense crescent. These signs include homogenous or diffuse heterogeneity of a mural thrombus, a low attenuation periluminal halo pattern, a smaller amount of thrombus and intrathrombotic calcification, an eccentric lumen with a thin wall, an eccentric lumen without a thrombus between the lumen and outer wall, and a focal transverse outpouching of the aortic wall as an aortic bleb(Fig. 9). Additional signs include focal discontinuity of the calcified rim, a thin posterior wall on lumbar osteophyte, wide draping of the aorta over the adjacent vertebral body, and obliteration of the anterior or lateral border of the psoas muscle.12)42)43)48)
Aortitis-Associated Aneurysms
Takayasu aortitis-induced aneurysm
An aortic aneurysm can develop as a complication of Takayasu aortitis. This complication is not common, but has been recently recognized. The reported incidence ranged from 10% to 48%.49)50) The common locations of a Takayasu aortitis-induced aortic aneurysm are a descending thoracic and thoracoabdominal aorta. The morphology of the aneurysm is usually extensive and fusiform. Aortic aneurismal dilatation can occur as post-stenotic dilatation in cases of chronic Takayasu aortitis. However, not all aneurysms are accompanied by aortic stenosis.49) As a sign of chronic Takayasu aortitis, transmural calcification is common in this type of aneurysm. This calcification is commonly observed as an amorphous patchy shape in a three-dimensional reconstruction image such as a volume rendering(Fig. 11). Since aortic rupture is a fatal complication of Takayasu aortitis, the observation of a secondary aneurysm might be a poor prognostic factor.51) The risk of rupture of a Takayasu aortitis-induced aneurysm is lower than for a conservative atherosclerotic aortic aneurysm.49) However, the usual treatment regimen for Takayasu aortitis consists of steroids, which can weaken the aortic wall and subsequently increase the risk of an aneurismal rupture.50)
Infected aortic aneurysm
An infected aortic aneurysm is defined as an "any aortic dilatation of an infectious origin regardless of its size or pathogen".52) Due to the infectious etiology, an infected aortic aneurysm is more common in elderly or debilitated people, especially in cases of subacute endocarditis or a septic state. The abdominal aorta is the most common site of an infected aortic aneurysm, although an infected aneurysm itself is more common in the femoral artery.53) An infected aortic aneurysm has been identified in 0.7-2.6% of all aortic aneurysms.12)54) Although a systemic inflammatory response can occur in an infected aortic aneurysm, there may no specific symptoms until the rupture occurs. In addition, 47% of cases are negative by blood culture. An infected aortic aneurysm has a higher mortality and morbidity than a conservative atherosclerotic aortic aneurysm due to these vague symptoms and signs, rapid progression, and high rupture risk.12)52)55)56) The overall mortality has been reported to be 16-67%.54)57-59)
The average diameter of an infected abdominal aortic aneurysm has been reported to be 5.4 cm. The major morphology is a saccular with lobulated contour. A fusiform aneurysm is also possible but less likely. An extensive mural thrombus may be observed. Calcification in the aneurismal wall or mural thrombus is not as common as with an atherosclerotic aneurysm. However, calcification cannot be used as a differentiating factor because it can be observed coincidentally. An infected aortitis usually precedes aneurismal dilatation and the aorta can appear normal in the early stage of disease progression. A periaortic soft tissue mass, stranding, and/or fluid has been reported to be the most commonly depicted finding of an infected aortic aneurysm.52) Air bubbles in the periaortic inflammatory granulation tissue, which are produced by gas-forming agents, may be noted but are not common. Periaortic inflammatory granulation tissue can result in adjacent vertebral body destruction and osteomyelitis(Fig. 12). CT or MRI can observe periaortic inflammation as a soft tissue mass with rim enhancement.12) Disruption of the intimal calcification, obscured aortic wall, paraaortic lymph node enlargement, and retroperitoneal hematoma at various stages are other image findings indicative of an infected aortic aneurysm.56)60-62)
Sueyoshi et al.63) suggested the very early signs of an infected aortic aneurysm or aortitis. Due to the inflammatory changes in the aortic wall, the attenuation value of periaortic fat increased in the MDCT image. Other investigators have reported the sudden appearance and rapid progression of abdominal aortic aneurysms during serial follow-up CT scans of patients with infected aortitis.64)65) In summary, a non-calcified saccular aneurysm, particularly with a lobulated contour in an unusual location that shows rapid expansion or development, an adjacent soft tissue mass, stranding and/or fluid, should be strongly suspected of being an infected aortic aneurysm.52)63)
Conclusions
Based on the development of radiology equipment such as MDCT or ultrasonography, the diagnostic accuracy of an aortic aneurysm has increased markedly along with the improvement in surgical and interventional treatment techniques. However, the mortality and morbidity rate of aortic aneurysms has not decreased significantly. More active approaches from internal medicine, radiology, molecular medicine and public healthcare are expected from more popular and convincing screening of high-risk groups, as well as from a more sensitive and accurate evaluation of aortic aneurysms.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download