Abstract
Background and Objectives
Ischemic injury is the most common and important cause of myocardial damage. Over past decades, a number of studies have identified a protective mechanism known as ischemic preconditioning, which can block or delay cell death from ischemic injury. Protein kinase C (PKC), especially the ε isoform has been proposed as a key factor in the signaling pathway of ischemic preconditioning. However, whether PKCε expression in cardiomyocytes can offer such protection from acute ischemia has not been explored.
Materials and Methods
To demonstrate a direct effect of PKCε expression, a lentiviral vector system was established. Using the lentiviral vector, PKCε was introduced to neonatal rat ventricular myocytes (NRVM) cultured under ischemic conditions, and also to adult rat myocardium subject to left coronary artery ligation.
Results
Compared to control, PKCε expression in cultured NRVM under ischemia resulted in preserved cell density and morphology, and a reduction in cell death (77.6±12.8% vs 58.1±7.2%, p<0.05). In adult rats, the infarcted area after coronary artery ligation was markedly reduced in myocardium injected with PKCε vector compared to control (11.4±5.3% vs 20.5±11.3%, p<0.01).
Figures and Tables
Fig. 1
Microscopic findings of neonatal rat ventricular myocyte (NRVM) cultured under various conditions. Control: NRVM cultured under normal condition, Ischemia: NRVM cultured under ischemic condition, Ischemia+TPA: NRVM cultured under ischemic condition treated with tetradecanoylphorbol 13-acetate (TPA), Ischemia+vector: NRVM culuted under ischemic condition treated with empty vetor, Ischemia+vector+TPA: NRVM cultured under ischemic condition treated with empty vector and TPA, Ischemia+PKC+TPA: NRVM cultured under ischemic condition treated with protein kinase Cε vector and TPA, PKC: protein kinase C.

Fig. 2
Comparison of cell death of cultured neonatal rat ventricular myocyte (NRVM) under various conditions. Number of cell death was determined by counting cells failing to exclude the trypan blue dye. Control: NRVM cultured under normal condition, Ischemia: NRVM cultured under ischemic condition, I+TPA: NRVM cultured under ischemic condition treated with tetradecanoylphorbol 13-acetate (TPA), I+PKC+TPA: NRVM cultured under ischemic condition treated with protein kinase Cε vector and TPA. *: p<0.05 between ischemia and ischemia+PKC+TPA, PKC: protein kinase C.
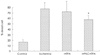
Fig. 3
Representative samples of transverse section of myocardium stained with phthalocyanin blue and triphenyltetrazolium chloride in control and treated rat heart, injected with PKCε lentiviral vector intramuscularly. Unstainted area (white color) represents infarcted myocardium. PKCε: protein kinase Cε.

Fig. 4
Bar graphs represent area of infarction between control and treated rat heart, injected with PKCε lentiviral vector intramuscularly. Control MI: rat heart injected with control buffer 4 days prior to left coronary artery ligation, PKCε MI: rat heart injected with PKCε vector 4 days prior to left coronary artery ligation. PKCε: protein kinase Cε, MI: myocardial infarction

Acknowledgments
We are deeply grateful to professor Yoshitaka Ono of Biosignal Research Center, Kobe University, for the generous gift of PKCε cDNA, which enables us to conduct this study.
References
1. Fibrinolytic Therapy Trialist's (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity from all randomized trials of more than one thousand patients. Lancet. 1994. 343:311–322.
2. Weaver WD, Simes RJ, Betriu A, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review. JAMA. 1997. 278:2093–2098.
3. Rogers WJ, Bowlby LJ, Chandra NC, et al. Treatment of myocardial infarction in the Unite States (1990 to 1993): observation from National Registry of Myocardial Infarction. Circulation. 1994. 90:2103–2114.
4. Reimer KA, Murry CE, Yamgawa I, Hill ML, Jennings RB. Four brief periods of ischemia cause no cumulative ATP loss or necrosis. Am J Physiol. 1986. 251:H1306–H1315.
5. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986. 74:1124–1136.
6. Simkhovich BZ, Pizyklenk K, Kloner RA. Role of protein kinase C as a cellular mediator of ischemic preconditioning: a critical review. Cardiovasc Res. 1998. 40:9–22.
7. Ping P, Zhang J, Qiu Y, et al. Ischemic preconditioning induces selective translocation of protein kinase C isoform ε and η in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997. 81:404–414.
8. Gray MO, Karliner JS, Mochly-Rosen D. A selective ε-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997. 272:30945–30951.
9. Miyanmae M, Rodriguez MM, Camcho SA, Diamond I, Mochly-Rosen D, Figueredo VM. Activation of protein kinase C correlates with a cardioprotective effect of regular ethanol consumption. Proc Natl Acad Sci USA. 1998. 95:8262–8267.
10. Springhorn JP, Claycomb WC. Preproenkephalin mRNA expression in developing rat heart and in cultured ventricular cardiac muscle cells. Biochem J. 1989. 258:73–78.
11. Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993. 88:1264–1272.
12. Murphy E. Primary and secondary signaling pathways in early and preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res. 2004. 94:7–16.
13. Goldberg M, Zhang HL, Steinberg SF. Hypoxia alters distribution of protein kinase C isoforms in neonatal rat ventricular myocytes. J Clin Invest. 1997. 99:55–61.
14. Kim HC, Kim H, Chung ST, et al. Cardioprotective effect of the ischemic preconditioning: its relation to activation of protein kinase C. Korean Circ J. 1999. 29:602–612.
15. Park SK, Kim HS, Son CS, Tockgo YC, Jeon YS. Expression of protein kinase C isoform mRNA in the developing rat heart. Korean Circ J. 1998. 28:1341–1349.
16. Son JW, Kim YD. Protein kinase C activation protects the cardiomyocytes from ischemic insults in adult, but not in neonatal rat heart. Korean Circ J. 2002. 32:689–696.
17. Goldberg M, Zhang HL, Steinberg SF. Hypoxia alters distribution of protein kinase C isoforms in neonatal rat ventricular myocytes. J Clin Invest. 1997. 99:55–61.
18. Inagaki K, Kihara Y, Hayashida W, et al. Anti-ischemic effect of a novel cardioprotective agent, JTV519, is mediated through specific activation of δ isoform of protein kinase C in rat ventricular myocardium. Circulation. 2000. 101:797–804.
19. Zhang HY, McPherson BC, Liu H, et al. Role of nitric-oxide synthase, free radicals, and protein kinase C delta in opiod-induced cardioprotection. J Pharmacol Exp Ther. 2002. 301:1012–1019.
20. Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994. 74:299–309.
21. Liu H, McPherson BC, Yao Z. Preconditioning attenuates apoptosis and necrosis: role of protein kinase C ε and- δ isoforms. Am J Physiol Heart Circ Physiol. 2001. 281:H404–H410.
22. Romano G, Micheli P, Pacilio C, Giordano A. Latest developments in gene transfer technology: achievment, perspectives, and controversies over therapeutic applications. Stem Cells. 2000. 18:19–39.
23. Romano G. Gene transfer in experimental medicine. Drug News Perspect. 2003. 16:267–276.
24. Okamura T, Miura T, Iwamoto H, et al. Ischemic preconditioning attenuates apoptosis through protein kinase C in rat hearts. Am J Physiol. 1999. 277:H1997–H2001.
25. Speechly-Dick ME, Mocanu MM, Yellon DM. Protein kinase C: its role in ischemic preconditioning in rat. Circ Res. 1994. 75:586–590.
26. Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M, Inserte J, Agullo L, Cabestrero A. The end-effectors of preconditioning protection against myocardial cell death secondary to ischemia-reperfusion. Cardiovasc Res. 2006. 70:274–285.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download