Abstract
Background and Objectives
Proteomics is a new technology that allows the detection and identification of several proteins at a given time in a sample. There are currently few reports concerned with the proteomic study of serum from patients during acute coronary syndrome. We performed proteomics to analyze the modifications in the serum protein map of patients with acute coronary syndrome (ACS).
Subjects and Methods
We investigated the serum from 12 patients who suffered with acute myocardial infarction (AMI), 12 patients with unstable angina (UA) and 13 age- and sex-matched patients as the control group. Two-dimensional electrophoresis, Coumassie staining and image analysis were performed. Mass spectrometry was performed to identify the selected spots.
Results
For the two-dimensional electrophoresis with using a pH range of 3 to 10, two different areas within the serum protein map were observed, and this showed differences between the groups. In area 1, three fibrinogen gamma chain isoforms were identified. All of them were increased in the serum from the AMI and UA patients when compared with the control group. In area 2, four fibrinogen beta chain isoforms were identified. Three isoforms of them were increased in the serum from the AMI and UA patients.
Figures and Tables
Fig. 1
Two-dimensional polyacrylamide gel electrophoresis of plasma in patients with acute coronary syndrome and control. There was no difference between the groups from computer-based image analysis. AMI: acute myocardial infarction, UA: unstable angina, CON: control, 1: albumin, 2: alpha-1-antitrypsin, 3: apolipoprotein A-I, Area 1: fibrinogen gamma, Area 2: fibrinogen beta.

Fig. 2
Patterns of fibrinogen gamma chain isoforms of plasma in patients with acute coronary syndrome and control. AMI: acute myocardial infarction, UA: unstable angina, CON: control, 1: isoform 1, 2: isoform 2, 3: isoform 3.
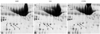
Fig. 3
Patterns of fibrinogen beta chain isoforms of plasma in patients with acute coronary syndrome and control. AMI: acute myocardial infarction, UA: unstable angina, CON: control, 1: isoform 1, 2: isoform 2, 3: isoform 3, 4: isoform 4.

Table 1
Baseline characteristics

Values are means±SD and No. of cases with percentage in parenthesis. ACS: acute coronary syndrome, AMI: acute myocardial infarction, UA: unstable angina, CON: control, M: male, F: female, DM: diabetes mellitus, TC: total cholesterol, TG: triglyceride, HDL: high density lipoprotein, LDL: low density lipoprotein
References
1. Oldgren J, Wallantin L, Grip L, Linder R, Norgaard BL, Siegbahn A. Myocardial damage, inflammation and thrombin inhibition in unstable coronary artery disease. Eur Heart J. 2003. 24:86–93.
2. Kim KY, Bae JH. The clinical usefulness of decreased mean platelet component concentration in patients with acute coronary syndrome. Korean Circ J. 2005. 35:240–246.
3. Lam L, Lind J, Semsarian C. Application of proteomics in cardiovascular medicine. Int J Cardiol. 2006. 108:12–19.
4. Chabers G, Lawrie L, Cash P, Murray GI. Proteomics: a new approach to the study of disease. J Pathol. 2000. 192:280–288.
5. Ahram M, Best CJ, Flaig MJ, et al. Proteomic analysis of human prostate cancer. Mol Carcinog. 2002. 33:9–15.
6. Srinivas PR, Srivastava S, Hanash S, Wright GL Jr. Proteomics in early detection of cancer. Clin Chem. 2001. 47:1901–1911.
7. Baek SY, Lee YM, Lee KH, Kim DS. Comparison of serum proteome maps of children with kawasaki disease. Korean J Pediatr. 2004. 47:81–89.
8. Lee CM, Kang SB, Cho YK, Choi H, Kim BR. Identification of biomarker for ovarian cancer by serum proteomic analysis using SELDI-TOF-MS. Korean J Gynecol Oncol. 2006. 17:147–156.
9. The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined. Eur Heart J. 2000. 21:1502–1513.
10. Wilkins MR, Sanchez JC, Gooley AA, et al. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1996. 13:19–50.
11. Wilkins MR, Pasquali C, Appel RD, et al. From proteins to proteomics: large scale protein identification by two-dimensional electrophoresis and amino acid analysis. Biotechnology. 1996. 14:61–65.
12. Anderson L, Seilharmer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997. 18:533–537.
13. Dunn MJ. Two dimensional gel electrophoresis of proteins. J Chromatogr. 1987. 418:145–185.
14. Gorg A, Obermaier C, Boguth G, et al. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000. 21:1037–1053.
15. Aebersold R. Mass spectrometry of proteins and peptides in biotechnology. Curr Opin Biotechnol. 1993. 4:412–419.
16. Gevaert K, Vandekerckhove J. Protein identification methods in proteomics. Electrophoresis. 2000. 21:1145–1154.
17. Patterson SD, Aebersold R. Mass spectrometric approaches for the identification of gel-separated proteins. Electrophoresis. 1995. 16:1791–1814.
18. White MY, Cordwell SJ, McCarron HC, et al. Proteomics of ischemia/reperfusion injury in rabbit myocardium reveals alterations to proteins of essential functional systems. Protemics. 2005. 5:1395–1410.
19. Yoon CH, Youn HJ, Chun HK, et al. Expression of heat shock protein 70 and association with activity of superoxide dismutase in ischemic preconditioning model of rabbit. Korean Circ J. 1999. 29:965–975.
20. Mateos-Caceres PJ, Garcia-Mendez A, Farre AL, et al. Proteomic analysis of plasma from patients during an acute coronary syndrome. J Am Coll Cardiol. 2004. 44:1578–1583.
21. Farrell DH, Thiagarajan P, Chung DW, Darie EW. Role of fibrinogen alpha and gamma chain sites in platelet aggregation. Proc Natl Acad Sci USA. 1992. 89:10729–10732.
22. Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993. 118:956–963.
23. Folsom AR, Wu KK, Shahar A, Davis CE. Association of hemostatic variables with prevalent cardiovascular disease and asymptomatic carotid artery atherosclerosis. Arterioscler Thromb. 1993. 13:1829–1836.
24. Meade TW. Fibrinogen in ischemic heart disease. Eur Heart J. 1995. 16:Suppl A. 31–35.
25. Wang XL, Wang J, McCredie RM, Wilcken DE. Polymorphism of factor V, factor VII, and fibrinogen genes: relevance to severity of coronary artery disease. Arterioscler Thromb Vasc Biol. 1997. 17:246–251.
26. Mannila MN, Eriksson P, Ericsson CG, Hamsten A, Silveira A. Epistatic and pleiotropic effects of polymorphisms in the fibrinogen and coagulation factor XIII genes on plasma fibrinogen concentration, fibrin gel structure and risk of myocardial infarction. Thromb Haemost. 2006. 95:420–427.
27. Bombeli T, Schwartz BR, Harlam JM. Adhesion of activated platelets to endothelial cells: evidencefor a GP IIb/IIIa dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3, integrin and GP Ib alpha. J Exp Med. 1998. 187:329–339.
28. Laffan MA. Fibrinogen polymorphisms and disease. Eur Heart J. 2001. 22:2224–2226.
29. Mannila MN, Silveira A, Hawe E, et al. Plasma fibrinogen concentration predicts the risk of myocardial infarction differently in various parts of Europe: effects of beta-fibrinogen genotype and environmental factors. Thromb Haemost. 2004. 92:1240–1249.
30. Mannila MN, Lovely RS, Kazmierczak SC, et al. Elevated plasma fibrinogen gamma concentration is associated with myocardial infarction: effects of variation in fibrinogen genes and environmental factors. J Thromb Haemost. 2007. 5:766–773.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download