Introduction
Stent thrombosis remains the primary cause of death after percutaneous coronary intervention(PCI). Despite improvement of PCI, stent thrombosis persists at a rate of 0.5-2% in elective cases, and up to 6% in patients with acute coronary syndromes.1)2) Stent thrombosis most often develops within the first 48 hours after the PCI, and rarely after a week of stent implantation.2) It almost always causes acute myocardial infarction(AMI) or sudden cardiac death.3-5) While very late stent thrombosis (VLST), occurring beyond 1 year, is not uncommon with the use of drug-eluting stents(DES), it is distinctly unusual with the use of bare-metal stents(BMS).3)4)6) We report a case of very late thrombosis of a bare-metal stent occurring 880 days after implantation.
Case
A 48-year-old male presented to our hospital with increasing exertional chest pain for a month on November 7, 2003. His chest pain was characterized by squeezing pattern and this was located in the substernal area and it radiated to his both shoulder. He had a managed for diabetes mellitus and hyperlipidemia. His initial blood pressure was 120/80 mmHg and pulse rate 88 beats per minute. The electrogram on admission showed T wave inversion in lead V1-4. Echocardiography revealed normal systolic function without any regional wall motion abnormality.
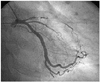
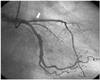
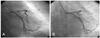
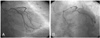
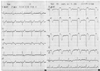
Cononary angiography revealed a subtotal occlusion of the proximal left anterior descending coronary artery(LAD)(Fig. 1), and an Arthos®(AMG, Faesfeld-Erle, Germany) stent(3.0×38 mm at 14 atm) was implanted with an excellent angiographic result(Fig. 2). He was discharged in stable condition and medicated with aspirin, clopidogrel, statin and oral hypoglycemic agents. Six months later, the patient was visit to our hospital for recurring exertional chest pain in May 2004. He had been taking clopidogrel(75 mg/day) for the initial 1 month post-stent implantation, but aspirin was continued. His coronary angiography revealed about 90% in-stent restenosis(ISR) of the previously stented segment of the proximal LAD(Fig. 3A), and the cutting balloon angioplasty(CBA) was performed(Fig. 3B). Clopidogrel was given for 6 months, and then it was discontinued thereafter, while aspirin was continued. After the procedure, the patient underwent follow-up coronary angiography with an excellent angiographic result in December 2004 (Fig. 4A, B). The patient was completely asymptomatic until April 2006. On April 5, 2006, the patient visited to other hospital with sudden severe chest pain. His electrocardiogram(ECG) revealed ST-segment elevation and Q-wave in precordial leads V1-4(Fig. 5). The patient was diagnosed as ST-elevation acute myocardial infarction in the anteroseptal localization. So, fibrinolytic agent was administrated in other hospital. And then, he was transferred to our hospital one day later. His ECG still revealed ST-segment elevation and Q-wave in precordial leads V1-4, and his troponin I increased up to 34.5 ng/mL on April 6, 2006. The antithrombin III, protein C and protein S levels were 25 mg/dL, 77.51% and 91.86%, respectively, in within normal limits. The patient was given aspirin and clopidogrel and was also started on heparin and nitroglycerin infusions in the emergency room. On April 7, 2006, his ECG revealed normalization of ST-segment elevation with Q-wave. He was transferred to the cardiac catheterization laboratory on April 8, 2006. Coronary angiography revealed a thrombotic occlusion of the previously stented segment of the proximal LAD, with 30% ISR(Fig. 6). But, the patient did not undergo any further coronary procedure because of restoration of perfusion to distal TIMI 3 flow. The patient was discharged with aspirin, clopidogrel and warfarin. After the six-month, follow-up coronary angiography revealed no thrombus with good patency of previuos stented site and IVUS(Fig. 7) showed good expansion of the stent with mild intimal hyperplasia.
Discussion
Stent thrombosis is generally a fatal complication after PCI. Coronary stent has dramatically improved upon the acute procedural success and also reduced the restenosis rates observed with balloon angioplasty alone. Significant improvements in the prevention of stent thrombosis have been achieved through the technical development of stent implantations and the use of potent antiplatelet agents.3)7) Stent thrombosis is classified as either subacute stent thrombosis(SAT), occurring within 30 days, or as late stent thrombosis(LST), occurring beyond 30 days.4)8-10) While VLST is uncommon with the use of DESs, it is distinctly unusual with the use of BMSs.4) Since BMSs are known to be re-endothelialized within a few weeks after the procedure, this rapid endothelialization of BMSs makes LST exceedingly rare.3)10)11) However, delayed endothelialization associated with the implantation of a DES may increase the risk of LST.10)
In the recently published TAXUS V trial, the incidence of stent thrombosis in the bare-metal arm of the study group was 0.5% at 30 days, 0.2% at 6 months and 0% at 9 months.6) There have been no studies looking specifically at the determinants and incidence of VLST with the use of BMS. Presumed causes of BMS thrombosis, both early and late, include noncompliance with antiplatelet agents, exercise-induced procoagulant state, brachytherapy, small stent size and underdeployment of the stent.3)9)12)13) Furthermore, longer stent length, number of implanted stents, stent malapposition, residual dissections, reduced TIMI flow, gene polymorphisms, and resistance to the antiplatelet effects of acetylsalicylic acid(ASA) and potentially thienopyridines have been reported to increase the risk for stent thrombosis.1)3)7)9)10) Premature discontinuation of antiplatelet therapy is the most common precipitation factor of stent thrombosis10) and coronary brachytherapy is also the most important risk factor of late stent thrombosis in BMSs.3) We could not identify any potential explanations for this thrombotic event in our patient except for the possibility of relatively small stent size(3.0 mm), longer stent length(38 mm) and resistance to antiplatelet agents, but IVUS in follow-up period, showed good expansion and apposition and our patient was persistently compliant with his drugs. Stent thrombosis of our patient may be new plague rupture or erosion in previuos stented segment.
The management of VLST is similar to that of SAT and LST, and consists of the restoration of perfusion to distal TIMI 3 flow - most commonly by a variety of percutaneous techniques such as balloon angioplasty, AngioJet®(Possis Medical, Inc., Minneapolis, Minnesota) thrombectomy, or re-stenting with subsequent long-term dual antiplatelet therapy.4) Our patient did not undergo any other coronary procedure because of restoration of perfusion to distal TIMI 3 flow. In other words, thrombolytic therapy was successful in restoration of perfusion.
The optimum duration of antiplatelet therapy for patients with coronary artery stents still remains to be determined. In the era of BMSs, it has been customary to prescribe dual antiplatelet therapy for the duration of 1 month followed by the indefinite treatment with ASA alone.3) More recently, randomized clinical trials tested the benefit of extended(9-12 months) dual anti-platelet therapy compared with treatment with ASA alone. Our report shows that stent thrombosis may arise as late as 880 days after performing successful PCI, even when treating this patient with antiplatelet agents. Therefore, clinicians should be concerned about the possibility of VLST in those patients who have undergone BMS implantation. Most importantly, education regarding the importance of compliance with combination anti-platelet therapy needs to be emphasized. In addition, careful attention should be paid to assure adequate high-pressure inflation during deployment.13) Further large-scaled studies are needed to determine the optimal combination and duration for antiplatelet therapy that should be used to prevent these serious thrombotic events.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download