Abstract
Background and Objectives
Nitric oxide (NO) is thought to have antiatherosclerotic properties. On the other hand, NO activity is reduced in patients with metabolic syndrome, and endothelial dysfunction is an important early sign of atherosclerosis in patients with metabolic syndrome. The aim of this study was to investigate the effect of pioglitazone on the endothelial function in terms of the plasma NOx (combined nitrate/nitrite), the circulating inflammatory markers and the autonomic nervous system.
Subjects and Methods
We randomized 40 subjects with metabolic syndrome, and they were assigned to receive 15 mg of pioglitazone per day (the PIO group, n=21) during 12 weeks or they were placed in the placebo group (the PLA group, n=19). We estimate the endothelial function by performing vascular ultrasound. The plasma NOx levels, the levels of the inflammatory markers and the GRK2 levels were measured.
Results
After 12 weeks of therapy, flow mediated dilation (FMD) was improved in the PIO group (from 6.7±6% to 11.7±5%, respectively: p<0.05), but not in the PLA group. The level of plasma NOx was increased in the PIO group (from 67.7±30 nmol/dL to 92.9±41 nmol/dL, respectively: p<0.001), but not in the PLA group. The plasma levels of hsCRP and IL-6 dropped significantly (from 2.6±2.3 mg/L to 1.2±1.3 mg/L and 1.7±2.1 pg/mL to 0.7±0.5 pg/mL, respectively: p<0.05) in the PIO group, but not in the PLA group. The levels of GRK2 (the PLA group from 0.0061±0.0023 ng to 0.0075±0.0031 ng, and the PIO group from 0.0024±0.002 ng to 0.0015±0.001 ng, p=ns) didn't dropped significantly.
Conclusion
Administration of PPAR-γ agonist in patients suffering with metabolic syndrome improves their endothelial function, enhances the production of NOx and reduces the proinflammatory markers, but this is not related to sympathetic regulation. PPAR-γ agonist may be able to modulate the progression of atherosclerosis.
Figures and Tables
 | Fig. 1Changes of lipid profiles. This bar Graphs showed the percent changes after treatment. Significant decrease in total cholesterol and triglyceride in pioglitazone group compared with placebo group. In contrast, no significant changes were observed in LDL-cholesterol, HDL-cholesterol and lipoprotein a. TG: triglyceride, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol, Lp (a): lipoprotein a. |
 | Fig. 2Changes of inflammatory markers. By inflammatory markers, hsCRP was reduced in pioglitazone group, but not in placebo group after 12 weeks of treatment. IL-6, another inflammatory markers, a regulator of CRP released from the hepatocytes, were also reduced significantly in pioglitazone group. hsCRP: high sensitivity C-reactive protein, IL-6: interleukin-6, NS: not significant. |
 | Fig. 3Changes of nitric oxide. The plasma Nox (combined nitrate/nitrite) level were increased significantly in pioglitazone group, but not in placebo group after 12 weeks of treatment, NS: not significant. |
 | Fig. 4Changes of GRK2 levels. No differences in the GRK2 levels of lymphocytes between the two groups of individuals. GRK2: G protein-coupled receptor kinases 2, NS: not significant. |
 | Fig. 5ΔFMD after 12 weeks of treatment. The changes of FMD after 12 weeks treatment were enhanced in pioglitazone group not in placebo group. ΔFMD: delta changes of flow-mediated dilatation. |
 | Fig. 6Changes of FMD. Looking at some results from vascular function in the two groups of patients on the two graphs here, the graph on the left side looks at endothelium-dependent vascular function and the graph on the right side shows the results from the endothelium-independent vascular function. Individuals in the pioglitazone group have significantly greater endothelium-dependent vascular dysfunction than individuals in the placebo group. There were no differences in endothelium-independent vascular function between the two groups of individuals. FMD: flow-mediated dilatation. |
Table 3
Multivariate logistic regression analysis with changes of FMD (ΔFMD≥2.1) dependent variables
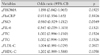
CI: confidence interval, ΔHOMA: changes of homeostatic model assessment index, ΔhsCRP: changes of high-sensitivity C-reactive protein, ΔNO: changes of nitric oxide, ΔIL-6: changes of interleukin-6, ΔTC: changes of total cholesterol, ΔTG: changes of triglyceride, ΔLDL-C: changes of low density lipoprotein cholesterol, ΔHDLC: changes of high density lipoprotein cholesterol
References
1. Yoo TW, Sung KC, Kim YC, et al. The relationship of the hypertension, insulin resistance, and metabolic syndrome in the serum uric acid level. Korean Circ J. 2004. 34:874–882.
2. Matsuoka H. Endothelial dysfunction associated with oxidative stress in human. Diabetes Res Clin Pract. 2001. 54:Suppl. S65–S72.
3. Sidhu JS, Cowan D, Kaski JC. The effects of rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, on marker of endothelial cell activation, C-reactive protein, and fibrinogen levels in non-diabetic coronary artery disease patients. J Am Coll Cardiol. 2003. 42:1757–1763.
4. Zeng G, Nystrom FH, Ravichandran LV, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000. 101:1539–1545.
5. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middleaged men. JAMA. 2002. 288:2709–2716.
6. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
7. Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl. 1968. 97:77–89.
8. Sorensen KE, Celermajer DS, Spiegelhalter DJ, et al. Non-invasive measurement of human endothelium-dependent arterial responses: accuracy and reproducibility. Br Heart J. 1995. 74:247–253.
9. Son JW, Koh KK, You SM, et al. Effects of simvastatin alone or combined with ramipril on nitric oxide bioactivity and inflammation markers in hypercholesterolemic patients. Korean Circ J. 2003. 33:1053–1059.
10. Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. Br J Pharmacol. 2000. 129:823–834.
11. Avena R, Mitchell HE, Nylen ES, Curry DM, Sidawy AN. Insulin action enhancement normalises brachial artery vasoactivity in patients with peripheral vascular disease and occult diabetes. J Vasc Surg. 1998. 28:1024–1032.
12. Festa A, D'Agostino R Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome. Circulation. 2000. 102:42–47.
13. Marx N, Libby P, Plutzky J. Peroxisome proliferator-activated receptors (PPARs) and their role in the vessel wall: possible mediators of cardiovascular risk? J Caridovasc Risk. 2001. 8:203–210.
14. Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferatoractivated receptor-gamma activators. Circulation. 2000. 101:235–238.
15. Freed MI, Ratner R, Marcovina SM, et al. Effects of rosiglitazone alone and in combination with atorvastatin on the metabolic abnormalities in type 2 diabetes mellitus. Am J Cardiol. 2002. 90:947–952.
16. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002. 51:2796–2803.
17. Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator activated receptor agonists. Diabetes. 2005. 54:2460–2470.
18. Boyle PJ, King AB, Olansky L, et al. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Ther. 2002. 24:378–396.
19. Hashimoto H, Kitagawa K, Hougaku H, et al. C-reactive protein is an independent predictor of the rate of increase in early carotid atherosclerosis. Circulation. 2001. 104:63–67.
20. Samaha FF, Szapary PO, Iqbal N, et al. Effects of rosiglitazone on lipids, adipokines, and inflammatory markers in nondiabetic patients with low HDL-C and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006. 26:624–630.
21. Caballero AE, Saouaf R, Lim SC, et al. The effects of troglitazone, an insulin-sensitizing agent, on the endothelial function in early and late type 2 diabetes: a placebo-controlled randomized clinical trial. Metabolism. 2003. 52:173–180.
22. Wang TD, Chen WJ, Lin JW, Chen MF, Lee YT. Effects of rosiglitazone on endothelial function, C-reactive protein and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2004. 93:362–365.
23. John S, Schlaich M, Langenfeld M, et al. Increased bioavailability of nitric oxide after lipid-lowering therapy in hypercholesterolemic patients: a randomized, placebo-controlled, double-blind Study. Circulation. 1998. 98:211–216.
24. Wennmalm A, Benthin G, Edlund A, et al. Metabolism and excretion of nitric oxide in humans: an experimental and clinical study. Circ Res. 1993. 73:1121–1127.
25. Calnek DS, Mazzella L, Roser S, Roman J, Hart CM. Peroxisome proliferator-activated receptor γ ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol. 2003. 23:52–57.
26. Lefkowitz RJ. G protein-coupled receptor kinases. Cell. 1993. 74:409–412.
27. Rockman HA, Choi DJ, Akhter SA, et al. Control of myocardial contractile function by the level of beta-adrenergic receptor kinase 1 in gene-targeted mice. J Biol Chem. 1998. 273:18180–18184.
28. Gros R, Chorazyczewski J, Meek MD, Benovic JL, Ferguson SS, Feldman RD. G-protein-coupled receptor kinase activity in hypertension: increased vascular and lymphocyte G-protein receptor kinase-2 protein expression. Hypertension. 2000. 35:38–42.
29. Park SJ, Choic DJ, Kim CW. Hypertensive left ventricular hypertrophy: relation to β-adrenergic receptor kinase-1 (β-ARK1) in peripheral lymphocytes. J Hypertens. 2004. 22:1025–1032.
30. Jin BC, Park TJ, Kim EJ, et al. Novel protein interactions of Gprotein-coupled receptor kinase 5 (GRK5) searched with yeast two-hybrid system. Korean Circ J. 2002. 32:613–622.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download