Abstract
Platelets play a central role in the pathogenesis of atherothrombosis. Dual antiplatelet therapy with clopidogrel plus aspirin has been shown to reduce ischemic events in patients undergoing percutaneous coronary intervention (PCI) and stenting. Although dual antiplatelet therapy reduces the risk of cardiovascular episodes after PCIs, a sub-stantial number of incidents continue to occur. Many cardiologists have focused their attention to the relationships between the interindividual variability of platelet inhibition after aspirin or clopidogrel administration and major cardiac adverse events such as stent thrombosis. Recent evidence has suggested that “aspirin or clopidogrel resistance” is associated with poor health outcomes (recurrent atherothrombotic events and stent thrombosis) after drug eluting stent (DES) implantation. However, the current clinical guidelines do not support routine screenings for antiplatelet resistance because standardized objective screening has not yet been established. Thus, this review describes the antiplatelet therapy used in PCI and it outlines the mechanism, laboratory tests, clinical impact and treatment options for aspirin and clopidogrel resistance in the DES era.
Go to : 
References
3. Chae SC. Antiplatelet agents in high-risk patients with coronary artery disease. Korean Circ J. 2004; 34:23–7.

4. Antithrombotic Trialists Collaboration. Collaborative meta-ana-lysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002; 324:71–86.
5. Smith JB, Willis AL. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971; 231:235–7.

6. Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005; 366:1607–21.
7. Steinhubl SR, Berger PB, Mann JT 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002; 288:2411–20.
8. Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002; 105:1650–5.

9. Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001; 88:230–5.

10. Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003; 107:2908–13.
11. Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005; 45:246–51.

13. Wilentz JR, Sanborn TA, Haudenschild CC, Valeri CR, Ryan TJ, Faxon DP. Platelet accumulation in experimental angioplasty: time course and relation to vascular injury. Circulation. 1987; 75:636–42.

14. Lev EI, Alviar CL, Arikan ME, et al. Platelet reactivity in patients with subacute stent thrombosis compared with non-stent-related acute myocardial infarction. Am Heart J. 2007; 153:41. e1–6.

15. Adams PC, Badimon JJ, Badimon L, Chesebro JH, Fuster V. Role of platelets in atherogenesis: relevance to coronary arterial restenosis after angioplasty. Cardiovasc Clin. 1987; 18:49–71.
16. Bae Y, Jeong MH, Kim NH, et al. The effects of antiplatelet agents in preventing coronary stent restenosis. Korean Circ J. 1999; 29:357–65.

17. Choe BR Lee CW, Park SW. Late stent thrombosis associated with late stent malapposition after drug-eluting stenting: a case report. Korean Circ J. 2006; 36:472–5.

18. Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004; 364:583–91.

19. Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004; 350:221–31.

20. Kastrati A, Dibra A, Eberle S, et al. Sirolimus-eluting stents vs paclitaxel-eluting stents in patients with coronary artery disease: meta-analysis of randomized trials. JAMA. 2005; 294:819–25.

21. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006; 48:193–202.
22. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006; 113:e166–286.
23. Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets: I. acetylation of a particulate fraction protein. J Clin Invest. 1975; 56:624–32.

24. Schwartz L, Bourassa MG, Lesperance J, et al. Aspirin and dipy-ridamole in the prevention of restenosis after percutaneous transluminal coronary angioplasty. N Engl J Med. 1988; 318:1714–9.

25. Conley PB, Delaney SM. Scientific and therapeutic insights into the role of the platelet P2Y12 receptor in thrombosis. Curr Opin Hematol. 2003; 10:333–8.

26. Daniel JL, Dangelmaier C, Jin J, Ashby B, Smith JB, Kunapuli SP. Molecular basis for ADP-induced platelet activation: I. Evidence for three distinct ADP receptors on human platelets. J Biol Chem. 1998; 273:2024–9.
27. Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004; 113:340–5.

28. Geiger J, Brich J, Honig-Liedl P, et al. Specific impairment of human platelet P2Y (AC) ADP receptor-mediated signaling by the antiplatelet drug clopidogrel. Arterioscler Thromb Vasc Biol. 1999; 19:2007–11.
29. Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation: II. the P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998; 273:2030–4.
30. Gent M, Blakely JA, Easton JD, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Lancet. 1989; 1:1215–20.

31. Hass WK, Easton JD, Adams HP Jr, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. N Engl J Med. 1989; 321:501–7.

32. Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting: the full anticoagulation versus aspirin and ticlopidine (fantastic) study. Circulation. 1998; 98:1597–603.
33. Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med. 1998; 339:1665–71.

34. Cattaneo M, Winocour PD, Somers DA, et al. Effect of ticlopidine on platelet aggregation, adherence to damaged vessels, thrombus formation and platelet survival. Thromb Res. 1985; 37:29–43.

35. Chea GS, Yoo HS, Jee JH, et al. Two cases of ticlopidine-induced neutropenia in patients with cardiovascular disease. Korean Circ J. 1998; 28:280–3.

36. Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med. 2003; 3:113–22.
37. Muller C, Buttner HJ, Petersen J, Roskamm H. A randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary-artery stents. Circulation. 2000; 101:590–3.

38. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001; 345:494–502.

39. Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001; 358:527–33.

40. Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics. JAMA. 2005; 294:1224–32.
41. Michos ED, Ardehali R, Blumenthal RS, Lange RA, Ardehali H. Aspirin and clopidogrel resistance. Mayo Clin Proc. 2006; 81:518–26.

42. Chen WH, Lee PY, Ng W, Tse HF, Lau CP. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol. 2004; 43:1122–6.

43. Cuisset T, Frere C, Quilici J, et al. Beneficial effect of a loading dose of 600 mg of clopidogrel on platelet parameters in patients admitted for acute coronary syndrome. Arch Mal Coeur Vaiss. 2006; 99:889–93.
44. Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003; 41:961–5.

45. Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis. J Am Coll Cardiol. 2005; 46:1827–32.

46. Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004; 109:3171–5.

47. Samara WM, Bliden KP, Tantry US, Gurbel PA. The difference between clopidogrel responsiveness and posttreatment platelet reactivity. Thromb Res. 2005; 115:89–94.

48. Steinhubl SR, Talley JD, Braden GA, et al. Point-of-care measured platelet inhibition correlates with a reduced risk of an adverse cardiac event after percutaneous coronary intervention. Circulation. 2001; 103:2572–8.

49. Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Catheter Cardiovasc Interv. 2007; 69:334–40.

50. Schroeder WS, Ghobrial L, Gandhi PJ. Possible mechanisms of drug-induced aspirin and clopidogrel resistance. J Thromb Thrombolysis. 2006; 22:139–50.

51. Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001; 345:1809–17.

52. Cipollone F, Rocca B, Patrono C. Cyclooxygenase-2 expression and inhibition in atherothrombosis. Arterioscler Thromb Vasc Biol. 2004; 24:246–55.

53. Rocca B, Secchiero P, Ciabattoni G, et al. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and charac-terizes newly formed platelets. Proc Natl Acad Sci U S A. 2002; 99:7634–9.

54. Macchi L, Christiaens L, Brabant S, et al. Resistance in vitro to low-dose aspirin is associated with platelet PlA1 (GP IIIa) polymorphism but not with C807T (GP Ia/IIa) and C-5T Kozak (GP Ibalpha) polymorphisms. J Am Coll Cardiol. 2003; 42:1115–9.
55. Halushka MK, Walker LP, Halushka PV. Genetic variation in cyclooxygenase 1: effects on response to aspirin. Clin Pharmacol Ther. 2003; 73:122–30.

56. Cambria-Kiely JA, Gandhi PJ. Aspirin resistance and genetic polymorphisms. J Thromb Thrombolysis. 2002; 14:51–8.
57. Csiszar A, Stef G, Pacher P, Ungvari Z. Oxidative stress-induced isoprostane formation may contribute to aspirin resistance in platelets. Prostaglandins Leukot Essent Fatty Acids. 2002; 66:557–8.

58. Serebruany VL, Malinin AI, Jerome SD, et al. Effects of clopidogrel and aspirin combination versus aspirin alone on platelet aggregation and major receptor expression in patients with heart failure. Am Heart J. 2003; 146:713–20.
59. Szczeklik A, Musial J, Undas A, et al. Inhibition of thrombin generation by aspirin is blunted in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1996; 16:948–54.

60. Andersen K, Hurlen M, Arnesen H, Seljeflot I. Aspirin nonresponsiveness as measured by PFA-100 in patients with coronary artery disease. Thromb Res. 2002; 108:37–42.

61. Buchanan MR, Brister SJ. Individual variation in the effects of ASA on platelet function: implications for the use of ASA clinically. Can J Cardiol. 1995; 11:221–7.
62. Hurlen M, Seljeflot I, Arnesen H. The effect of different antithrombotic regimens on platelet aggregation after myocardial infarction. Scand Cardiovasc J. 1998; 32:233–7.

63. Grotemeyer KH. Effects of acetylsalicylic acid in stroke patients: evidence of nonresponders in a subpopulation of treated patients. Thromb Res. 1991; 63:587–93.

64. Zhao L, Bath P, Heptinstall S. Effects of combining three different antiplatelet agents on platelets and leukocytes in whole blood in vitro. Br J Pharmacol. 2001; 134:353–8.

65. Wang JC, Aucoin-Barry D, Manuelian D, et al. Incidence of aspirin nonresponsiveness using the Ultegra Rapid Platelet Function Assay-ASA. Am J Cardiol. 2003; 92:1492–4.

66. Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J. 2006; 27:647–54.

67. Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126:234S–64S.
68. FitzGerald GA, Oates JA, Hawiger J, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983; 71:676–88.

69. Reilly IA, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood. 1987; 69:180–6.

70. Mirkhel A, Peyster E, Sundeen J, et al. Frequency of aspirin resistance in a community hospital. Am J Cardiol. 2006; 98:577–9.

71. Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation. 2001; 103:363–8.

72. Ghuysen A, Lambermont B, Dogne JM, et al. Effect of BM-573 [ N-terbutyl-N'-[2-(4'-methylphenylamino)-5-nitro-benzenesulfo-nyl] urea], a dual thromboxane synthase inhibitor and thromboxane receptor antagonist, in a porcine model of acute pulmonary embolism. J Pharmacol Exp Ther. 2004; 310:964–72.
73. Lau WC, Waskell LA, Watkins PB, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation. 2003; 107:32–7.

74. Lau WC, Gurbel PA, Watkins PB, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation. 2004; 109:166–71.

75. Serebruany VL, Midei MG, Malinin AI, et al. Absence of interaction between atorvastatin or other statins and clopidogrel. Arch Intern Med. 2004; 164:2051–7.

76. Saw J, Steinhubl SR, Berger PB, et al. Lack of adverse clopidogrel-atorvastatin clinical interaction from secondary analysis of a randomized, placebo-controlled clopidogrel trial. Circulation. 2003; 108:921–4.

77. Fontana P, Dupont A, Gandrille S, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003; 108:989–95.
78. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet aggregation according to body mass index in patients undergoing coronary stenting: should clopidogrel loading-dose be weight adjusted? J Invasive Cardiol. 2004; 16:169–74.
79. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005; 54:2430–5.

80. Cuisset T, Frere C, Quilici J, et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost. 2006; 4:542–9.

81. Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol. 2005; 45:1392–6.

82. von Beckerath N, Taubert D, Pogatsa-Murray G, Schomig E, Kastrati A, Schomig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel. Circulation. 2005; 112:2946–50.

83. Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Scias-cio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention. Circulation. 2005; 111:2099–106.

84. von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J. 2007. [Epub ahead of print].

85. Tanaka T, Ishikawa T, Hagiwara M, Onoda K, Itoh H, Hidaka H. Effects of cilostazol, a selective cAMP phosphodiesterase inhibitor on the contraction of vascular smooth muscle. Pharmacology. 1988; 36:313–20.
86. Byun KH, Shim JK, Min SJ, Jeon SJ, Kim JH. The multiple combined oral platelet inhibitors of aspirin and cilostazol after PCI. Korean Circ J. 2004; 34:443–50.
87. Yoon MH, Tahk SJ, Lian ZX, et al. The effects of two-month combination therapy of cilostazol and aspirin after intracoronary stenting. Korean Circ J. 2000; 30:927–36.

88. Lee SW, Park SW, Hong MK, et al. Triple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosis. J Am Coll Cardiol. 2005; 46:1833–7.
89. Dalby M, Montalescot G, Bal dit Sollier C, et al. Eptifibatide provides additional platelet inhibition in non-ST-elevation myocardial infarction patients already treated with aspirin and clopidogrel: results of the platelet activity extinction in non-Q-wave myocardial infarction with aspirin, clopidogrel, and eptifibatide (PEACE) study. J Am Coll Cardiol. 2004; 43:162–8.

90. Kastrati A, Mehilli J, Neumann FJ, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment. JAMA. 2006; 295:1531–8.
91. Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasu-grel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am Heart J. 2007; 153:66. e9–16.

92. Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006; 27:1038–47.

Go to : 
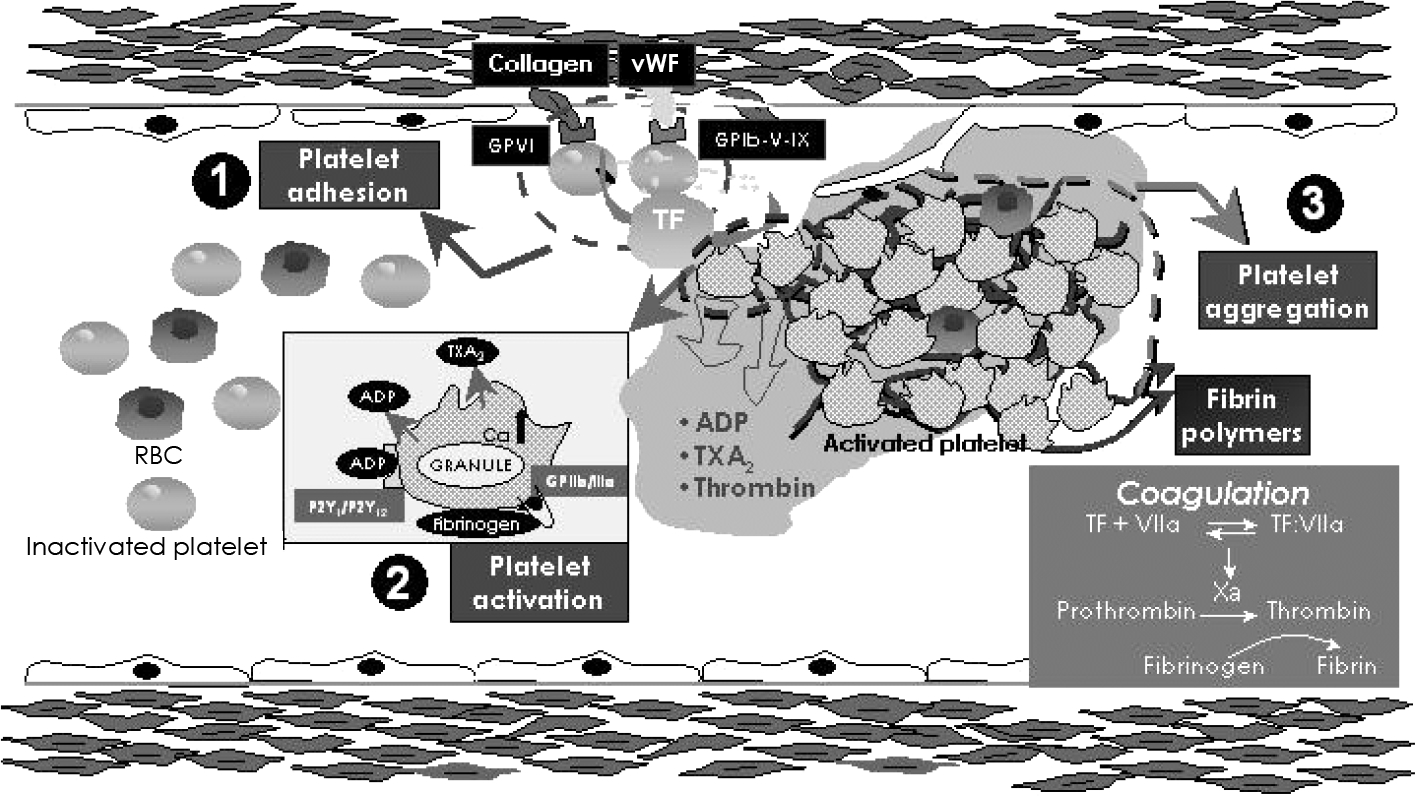 | Fig. 1.Platelet adhesion, activation and aggregation. ①: platelet adhesion is mediated by adhesive proteins such as von Willebrand factor (vWF). These adhesive proteins interact with platelet receptors such as glycoprotein Ib complex and glycoprotein VI, which also regulate platelet-leukocyte adhesion.②: platelets can be activated by adhesion to the arterial wall and by interacting with such circulatory agents as epinephrine, thrombin, serotonin, thromboxane A2 (TXA2) and adenosine diphosphate (ADP) via specific platelet surface receptors. Platelet activation leads to change of the platelet shape, from a smooth discoid contour into a speculated form. Following the shape change, platelet activation involves secretion of alpha and dense granules (ADP release) within the platelet. Platelet activation also induces phospholipase A2 activation that triggers arachidonic acid metabolism. Platelet cyclooxygenase 1 (COX-1) catalyzes the conversion of arachidonic acid to TXA2, which enhances platelet activation and vasoconstriction. ③: platelet activation leads to a conformational change in the glycoprotein IIb/IIIa receptor, converting the receptor into a form that can bind fibrinogen and link with other platelets (platelet aggregation). GP: glycoprotein, RBC: red blood cell, TF: tissue factor (Adapted and modified from reference 1). |
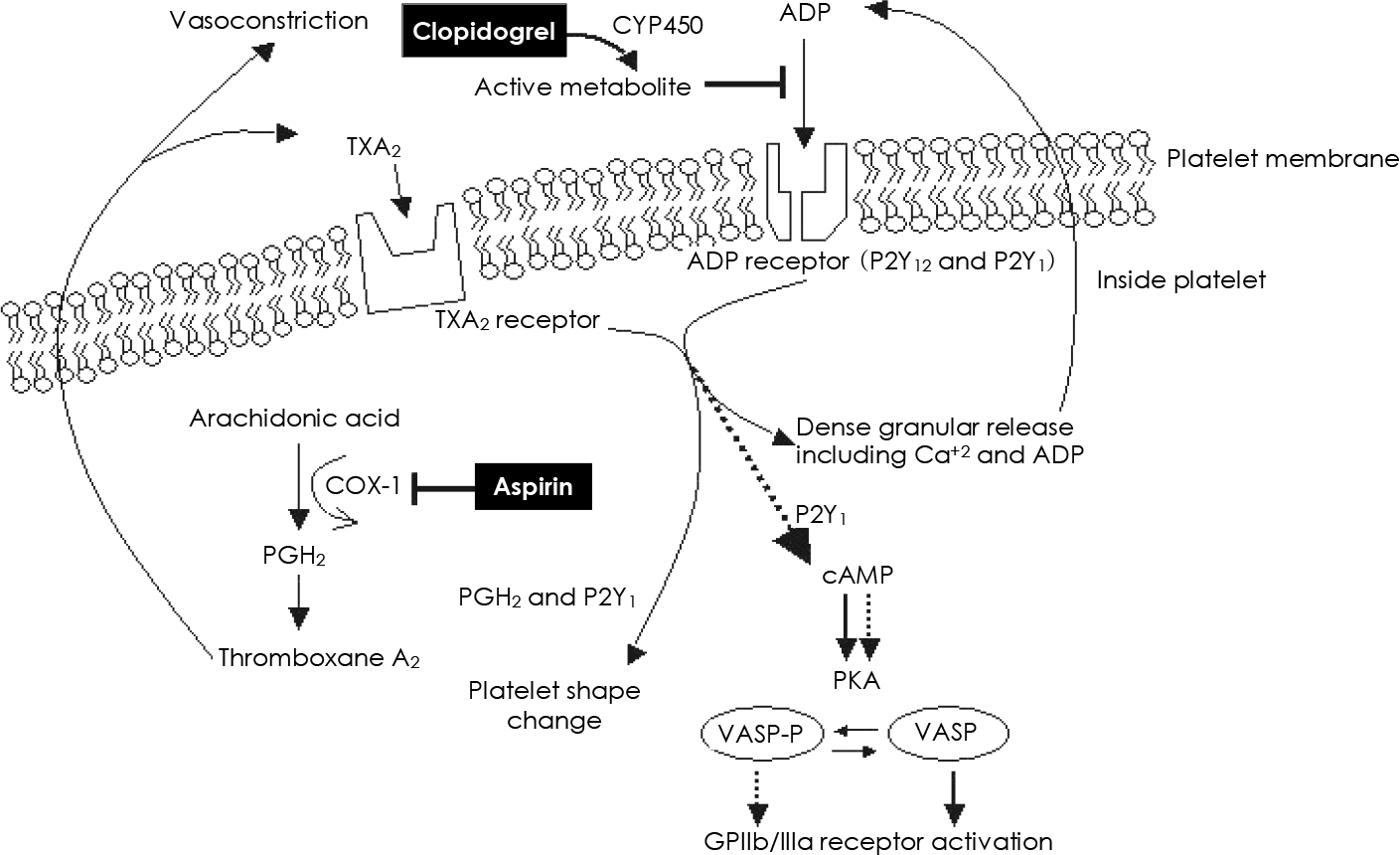 | Fig. 2.Schematic illustration of the pharmacologic sites of aspirin and clopidogrel. COX-1: cycloxygenase-1, PGH2: prostaglandin H2, TXA2: thromboxane A2, ADP: adenosine diphosphate, PKA: protein kinase-A, VASP: vasodilator stimulated phosphoprotein, cAMP: cyclic adenosine monophosphate, CYP450: cytochrome P450 (Adapted from reference 41). |
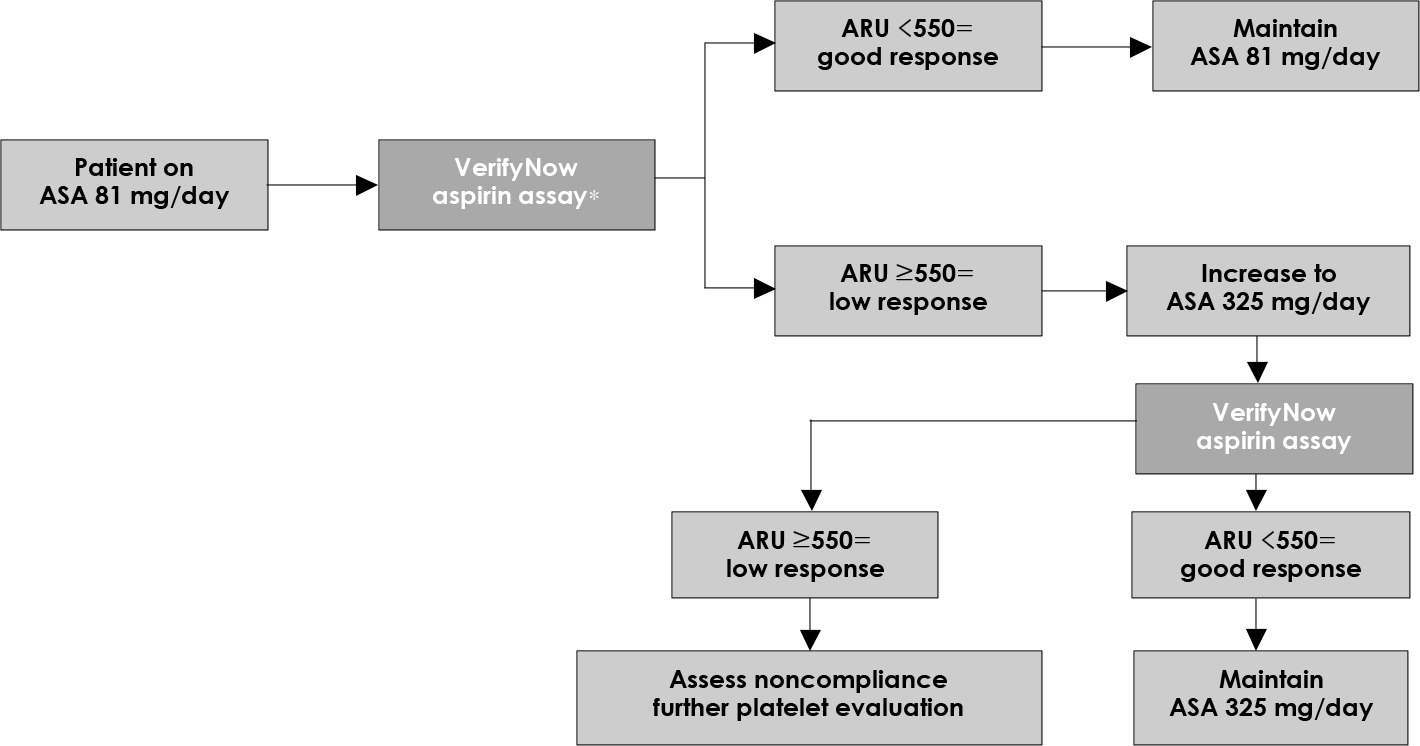 | Fig. 4.Critical pathway for aspirin (ASA) response with using a point-of-care system (VerifyNow Aspirin assay). ARU: aspirin reaction unit (Adapted from reference). |
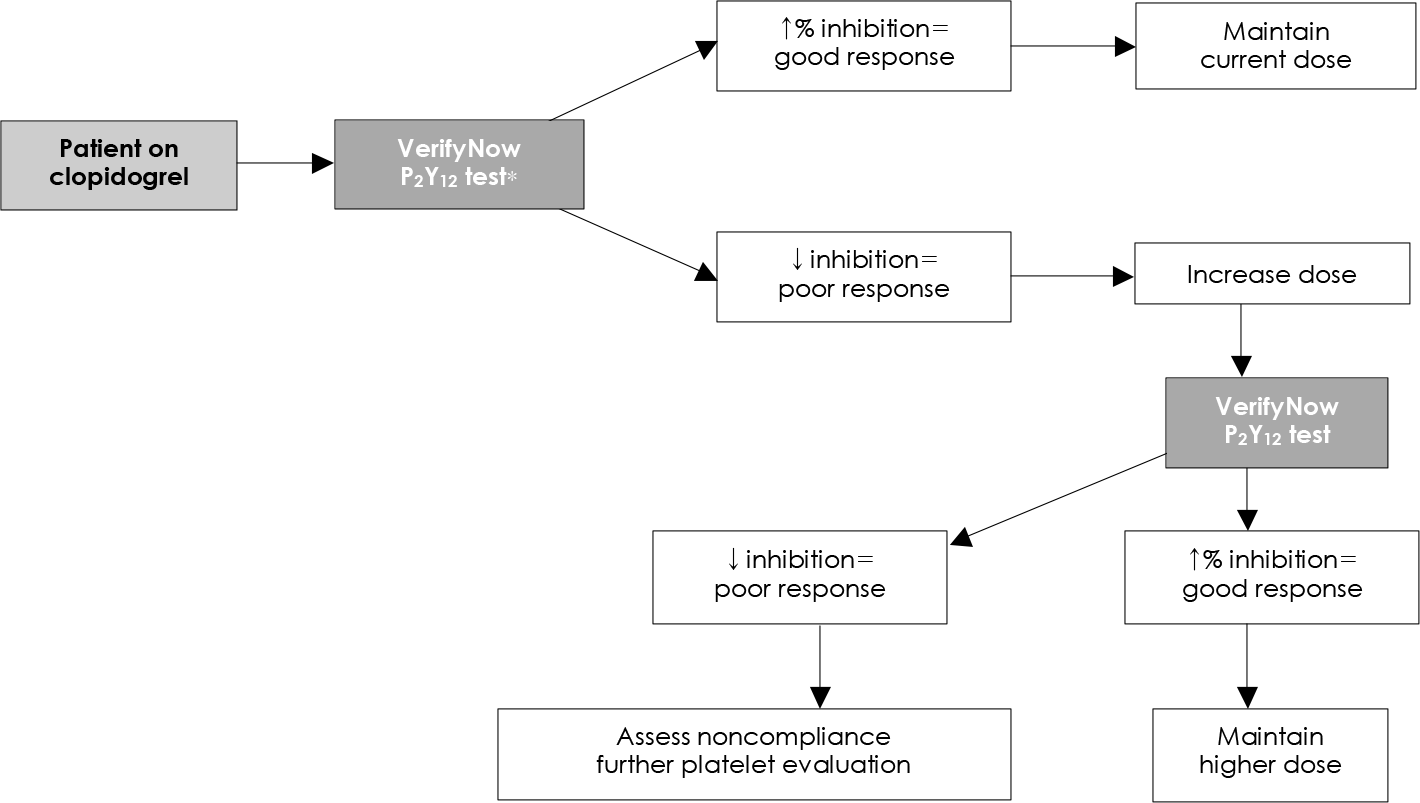 | Fig. 5.Critical pathway for clopidogrel response using a point-of-care system (VerifyNow P2 Y12 test) (Adapted from reference). |
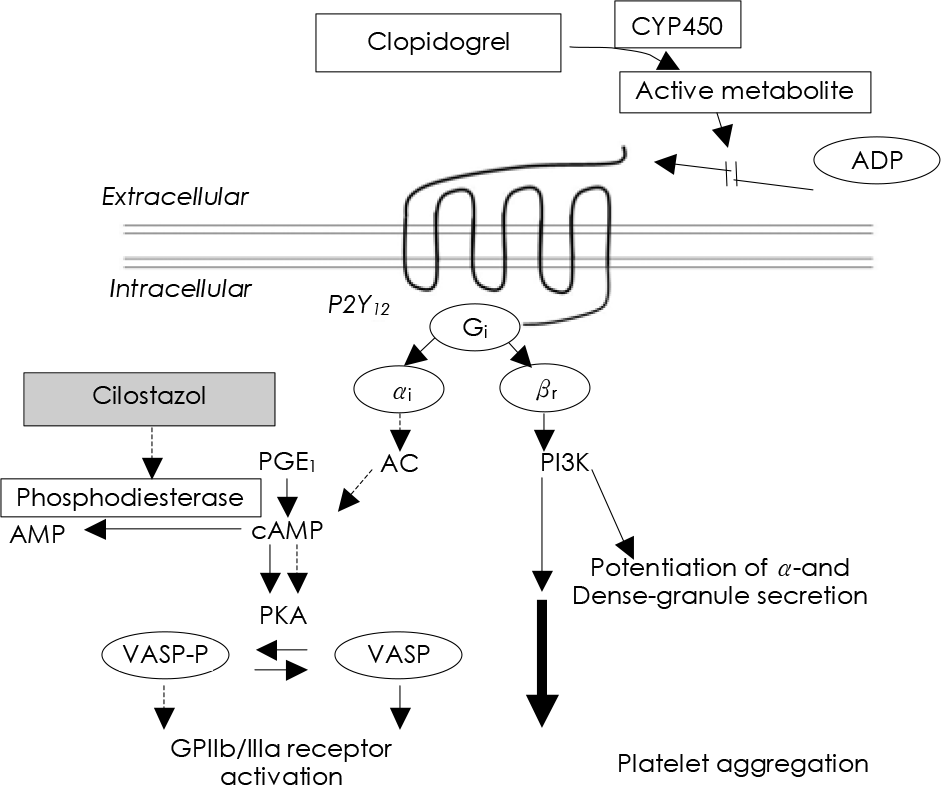 | Fig. 6.Proposed mechanism of improving clopidogrel resistance by cilostazol. ADP: adenosine diphosphate, PKA: protein kinase-A, VASP: vasodilator stimulated phosphoprotein, AC: adenylate cyclase, cAMP: cyclic adenosine monophosphate. PGE1: alprostadil (Adapted from reference). |
Table 1.
Possible mechanisms of aspirin resistance
Table 2.
Prospective studies regarding aspirin resistance
Table 3.
Laboratory assays for aspirin and clopidogrel resistance
Table 4.
Possible mechanisms of clopidogrel resistance
Table 5.
Prospective studies regarding clopidogrel resistance




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download