Introduction
Pulmonary embolism(PE) among pregnant and postpartum women remains a major cause of maternal morbidity and mortality. Deep vein thrombosis(DVT) usually starts in the calf veins, and may extend to the proximal veins and cause a pulmonary embolism.1)2) Available data show that the risk period for deep vein thrombosis and pulmonary embolism is greatest during the third trimester and postpartum.3)4) However, herein is report a rare case of a patient who suffered from deep vein thrombosis and pulmonary embolism during the first trimester.
Case
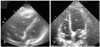
A 29-year-old woman in her 8th week of pregnancy was referred to our hospital due to progressive swelling in the lower extremities over a 2-week period, acute onset of dyspnea(1 hr) and pre-syncope. She had a 5-year history of hypothyroidism and daily Synthyroid(0.1 mg) administration. She had experienced a spontaneous abortion in the 12th week of pregnancy 1 year earlier. Otherwise, she had no significant past medical history and was taking no medication upon admission to the hospital. On examination, both her lower legs exhibited swelling and stasis, particularly the left lower leg. She weighed 87.6 kg and was 167 cm tall. Her blood pressure, heart rate and respiratory rate were 110/80 mmHg, 130 per minute and regular and 24 per minute, respectively. Arterial blood gas analysis on room air gave a pH of 7.43, PaCO2 30.9 mmHg, PaO2 56.5 mmHg, bicarbonate 20.0 mmol/L, oxygen saturation 90.6% and P (A-a)O2 30.5 mmHg. The prothrombin time, partial thromboplastin time and protein C were all within normal levels. Fibrin/fibrinogen degradation product and D-dimer values were 54.6 µg/mL and 6.7 mg/L, respectively, and the B-type natriuretic peptide(BNP) was 354.5 pg/mL. Serum titers of the anticardiolipin antibody(IgG, IgM) were within normal levels. Antinuclear and antineutrophil cytoplasmic autoantibodies(ANCA) were both negative. No factor V Leiden or prothrombin G20210A mutations were found. Deficiencies were observed in both the antithrombin III(18.0 mg/dl) and protein S activity(33%). ECG demonstrated sinus tachycardia and right axis deviation. A chest roentgenogram revealed mild hilar enlargement. A 2D echocardiogram revealed a normal left ventricular systolic function, dimension with a dilated right heart, an impaired right ventricular systolic function and an estimated pulmonary artery pressure of 55 mmHg(Fig. 1A). Venous ultrasonography demonstrated a left calf vein thrombosis, proximal vein thrombosis and incompressible posterior tibial veins(Fig. 2A-C). However, a ventilation perfusion (V/Q) lung scan and CT pulmonary angiography were not performed due to a labor-management dispute at our hospital, and because of concerns about the effects of radiation on the fetal development and the risk of developing breast cancer.5) The patient received thrombolytic therapy(tissue-type plasminogen activator: 50 mg for 1 hr, 50 mg for 2 hrs) followed by intravenous heparinization, and was closely monitored in the intensive care unit. She improved dramatically following the thrombolytic therapy, with a marked reduction in the dyspnea and significant improvement in her exercise capacity. Two weeks following the thrombolytic therapy, a 2D echocardiographic assessment revealed significant improvement in the right ventricular function, with reduction in the right ventricular dimension and an estimated pulmonary artery pressure of 37 mmHg(Fig. 1B). Her D-dimer level decreased to 0.4 mg/L. An obstetric assessment confirmed no evidence of fetal distress, with the baby continuing to progress. The patient responded very well over time and was continued on low molecular weight heparin(Fraxiparine®: nadroparin, 86 U/kg, SC, 2 times/day). Three weeks later, she made an uneventful recovery and was discharged with antepartum prophylaxis treatment(Fraxiparine® 3800 U, SC, 2 times/day). Finally, she delivered a normal, healthy infant at full-term(40 weeks).
Discussion
The overall incidence of deep vein thrombosis or pulmonary embolism during pregnancy has been reported as in 1 in 1000 to 1 in 2000 pregnancies. However, the relatively low incidence of venous thromboembolism (VTE) in Asians has been estimated, and may be related to a lower prevalence of genetic factors predisposing to VTE, such as factor V Leiden in Asians(0.5%) compared to Caucasians(5%).6) Several factors may be important in the development of VTE during pregnancy, such as hypercoagulability, venous stasis and vascular damage. Hypercoagulability results from increased levels of coagulation factors(VIII and fibrinogen) during normal pregnancy. Furthermore, around 40% of pregnancies acquire resistance to activated protein C. Reduction in protein S, the co-factor of protein C, is seen in normal pregnancy, and fibrinolysis is impaired by increased levels of plasminogen activator inhibitors 1 and 2, which are derived from the placenta.7)8) In addition, one or more heritable or acquired thrombophilias are found in at least 50% of VTE cases during pregnancy. Deficiencies of antithrombin, protein C and protein S are uncommon. Factor V Leiden mutation, activated protein C resistance and prothrombin G20210A mutation are relatively common, and have been reported to carry an increased risk of VTE.7)9) Vascular damage of pelvic vessels caused by delivery( vaginally or Caesarean section) probably contributes to postpartum venous thrombosis. Venous stasis begins by the end of first trimester, reaches a nadir at 36 weeks as a result of increased vein distensibility and compression of pelvic vessels by the gravid uterus, and takes about 6 weeks to return to the normal non-pregnant flow rates.10)11) The majority(80-90%) of DVTs in pregnancy develop in the left leg. Compression of the left iliac vein due to the right iliac artery where they cross may explain the preponderance of left leg DVT. The pregnancy specific risk factors that have been demonstrated include prolonged bed rest, advanced maternal age(over 35), family history of thrombosis, parity, previous thrombosis, thrombophilia, previous superficial phlebitis, pre-eclampsia, tobacco use and operative delivery.12) In this case, obesity, prolonged bed rest and thrombophilia(decreased antithrombin III and protein S activity) may have contributed to the pregnancy associated VTE.
The ventilation-perfusion scan and CT pulmonary angiography are the gold standards for the diagnosis of PE in both pregnant and non pregnant patients. Nevertheless, physicians are often reluctant to perform radiologic studies during pregnancy due to concerns over the effects of radiation on the fetal development. However, the estimated exposure of the fetus to radiation during these examinations is small. In most studies, exposure to radiation of less than 50,000 µGy(5 rad) has not been associated with significant risk of fetal injury.13) Therefore, many experts strongly recommend that venous thromboembolism is aggressively investigated, with definitive studies, whenever suspected in a pregnant patient. Presently, noninvasive echocardiographic and Doppler analysis of the right heart dimension and RV function neither definitively confirmation nor exclude suspected pulmonary embolism(PE). However, a typical echocardiographic picture of hemodynamically significant PE includes dilated, hypokinetic RV, an increased RV/LV ratio caused by interventricular septal bulging into the LV, dilated proximal pulmonary arteries, increased velocity of the jet of tricuspid regurgitation(usually in the range of 3-3.5 m/sec), a disturbed flow velocity pattern in the RV outflow tract and a dilated inferior vena cava(not collapsed on inspiration).14-16) The initial management of VTE confirmed during pregnancy necessitates immediate anticoagulation with unfractionated heparin or low molecular weight heparin(LMWH). According to available data, unless absolutely contraindicated, thrombolysis should be given to all patients with a massive PE (shock and/or hypotension). In patients with normal blood pressure, normal tissue perfusion and clinical or echocardiographic evidence of RV dysfunction(submassive PE), thrombolytic therapy may be given in the absence of contraindications. In our case, thrombolytic therapy, with immediate full dose anticoagulation, was decided for faster hemodynamic improvement compared to heparin alone, due to evidence of acute cor pulmonale, coinciding with high clinical suspicion of acute onset submassive PE, without previous cardiac or respiratory disease. She received intravenous heparin for 5 days, and Subcutaneous LMWH was then continued, with a fulldose regimen, for 3 weeks. At the time of discharge, the LMWH was reduced to a half-dose regimen, which was continued throughout the remainder of the pregnancy. Fortunately, she responded very well, without any serious side-effects.
Of the women with a history of VTE during pregnancy, the incidence of recurrence during subsequent pregnancies has been estimated as 4 to 15 percent.17)18) The risk of recurrent VTE is the same for patients with proximal DVT or PE, but the risk of a fatal PE is 2- to 3-fold higher after an episode of PE than of DVT.19) Therefore, most experts recommend that pregnant women at risk of VTE receive more aggressive prophylaxis than that traditionally recommended.
In conclusion, because of the gravity of maternal morbidity and mortality, the need for prolonged heparin therapy during pregnancy and prophylaxis during subsequent pregnancies, physicians should not be reluctant to perform definitive radiologic studies whenever VTE is suspected in a pregnant woman. Further studies will be required to make a confident recommendation in pregnancy associated VTE.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download