Abstract
Atherosclerosis is a generalized disorder that progresses very slowly. Early detection of atherosclerosis is very important to prevent cardiovascular disease such as myocardial infarction, stroke and sudden cardiac death. Various surrogate markers have recently been proposed for the early detection of atherosclerosis in asymptomatic patients who have one or more risk factors. Among them, biomarkers such as CRP, Interleukin, myeloperoxidase, fibrinogen, homocystein and lipoprotein (a) are established as predictors of atherothrobotic events in apparently healthy individuals. Although these novel biomarkers provide important information into the pathophysiology of atherosclerosis, no clear evidence exist that lowering the plasma level of these markers reduces the vascular risk. Imaging markers such as the carotid intima-media thickness and brachial arterial flow mediated vasodilation as assessed by ultrasound, coronary calcification as assessed by CT, and the pulse wave velocity and augmentation index as assessed by tonometry can visualize the arterial wall and directly measure the arterial function. These imaging markers are very useful clinical tools for detecting the early changes of vascular structure and also for predicting cardiovascular events, in addition to being more precise biomarkers in asymptomatic subjects.
The first clinical manifestation of atherosclerotic disease frequently is major cardiovascular disease such as myocardial infarction, stroke or sudden cardiac death; however, it often remains a clinically silent condition for many years.1)
Despite detection and treatment of the major traditional risk factors such as hypertension, hypercholesterolemia, diabetes and smoking, cardiovascular disease (CVD) has recently displayed an increased incidence and it has become one of most common causes of death in the Korean population. Early detection of the risk factors is necessary to decrease the morbidity and mortality of CVD.2) The traditional risk factors have been established to predict the CVD; however, they have limitations for predicting CVD. Thus, new surrogate markers of cardiovascular risk have been proposed, and they are believed to better detect patients who are at high risk than using only the traditional risk factors.3)4) Among them, the measures of subclinical arterial disease are the subject of growing interest because they may better represent the markers of CVD susceptibility. Angiography has been traditionally regarded as a gold standard technique for detecting arterial atherosclerosis by directly visualizing vessel stenosis.5) However, arterial stenosis reflects an advanced stage of atherosclerosis, so the early atherosclerosis cannot be detected by conventional angiography.
Other imaging modalities such as ultrasound, vascular CT and MRI are currently available for detecting early vascular atherosclerosis in patients who do not display any evidence of CVD; these modalities are reported to predict the occurrence of CVD more accurately than can the traditional risk factors. This paper focused on how to assess subclinical arterial disease with using various imaging systems and we review the clinical impact of such evaluation to improve both the management of patients' risk factors and the results of treatment.
B-mode ultrasound allows quantitative measurement of the carotid artery wall thickness. The definition of the intima-media thickness(IMT) is the distance from the upper margin of the intima to the media/adventitia echogenic interfaces(Fig. 1). Pignoli et al.6) firstly reported that the carotid intima-media thickness, as measured by B-mode ultrasound, was similar both in living patients and on autopsy findings. In their study, the double line pattern of the far wall was consistently found in normal or mild atherosclerotic lesion such as a fatty streak. In more advanced atherosclerotic lesion, the double line pattern was sometimes disrupted and more complex; these specimen showed evidence of a fibromuscular cap with a lipid and/or necrotic core; histologically, the intima showed focal areas of fibrotic smooth muscle cell proliferation, microcalcification and necrosis. Based on this article,2) many studies were performed to measure the carotid IMT as a marker of atherosclerosis and as a predictor of the vascular events that result from atherothrombosis.7-12)
Two methods have generally been used to measure carotid IMT. The first method is the overall mean maximum carotid IMT, i.e., the mean of the maximum thickness of 6 to 12 sites, and this is combined with measurement of carotid IMT that's performed in the common carotid artery(CCA), the carotid bifurcation and the near wall and/or far wall. The second method is only measurement of the CCA. Automated computerized operator-independent reading(Fig. 2) has recently become available and it is easy to use in daily practice, and this achieves excellent precision and reproducibility of IMT measurements in the common carotid artery.13)14) However, computerized measurement does not work as well on the carotid bifurcation and the internal carotid artery as it does on the common carotid because both former segments frequently include plaques that display disrupted interfaces when they are analyzed by the computerized program.13) Up to now, the IMT measurement procedure is not standardized as to where to measure as regards to the arterial wall, the far wall and/or the near wall, and as regards the arterial segment within the carotid tree. However, in most of the recent studies, the IMT is often measured and averaged along a longitudinal segment >1 cm in length of the proximal common carotid far wall, a site free of atherosclerotic lesion. This method showed higher reproducibility and accuracy, The IMT is not always a sign of atherosclerosis and it may represent hypertension-related hypertrophy.13) Alternatively, when the IMT is the average of several focal measures in the near and far walls of all the carotid trees, it can be considered as a surrogate marker of atherosclerosis, and especially for patients with established coronary artery disease; this is because atherosclerotic lesions, which are frequent in the bifurcation and internal carotid, are incorporated into its measurement.11)13)14) More recently, some investigator have tried to measure just the intima thickness. The improving image quality has allowed measurement of the intima,15)16) which represents a more accurate way to define preatherosclerotic lesion. Despite these methodological problems, there is evidence that an increased IMT, whatever the site of measure, is associated with an increased incidence of subsequent CVD in asymptomatic subjects.13)
There have been numerous studies that evaluated the correlation between carotid IMT and cardiovascular events(Table 1). Salonen et al.7) reported that for middle-aged men without known CVD, the 3-year risk of acute myocardial infarction increased by 11% with each 0.1 mm increase in the common carotid IMT, and the predictive value of the IMT remained significant after adjusting for age, smoking, blood pressure and the total and HDL cholesterol. In the Atherosclerosis Risk in Communities study(ARIC), the prevalence of coronary artery disease increased in parallel with the increasing IMT(ARIC),17) and an increased IMT above 1 mm at baseline, as compared with the IMT values below 1 mm, was associated with a fivefold(women) and twofold (men) increase in the age-adjusted, race-adjusted risk of coronary heart disease; this study was conducted over a period of 4-7 years on asymptomatic subjects. The Rotterdam study on asymptomatic subjects aged 55 years or older also confirmed that the risk of stroke and myocardial infarction over a mean follow-up duration of 2.7 years increased continuously with an increased IMT at baseline.18) The Cardiovascular Health Study(CHS) on asymptomatic elderly subjects has shown that the risk of myocardial infarction or stroke increased with the IMT, about fourfold between the quintile with the highest IMT and the quintile with the lowest IMT over a median follow-up duration of 6.2 years.19) For examining secondary prevention, in the Cholesterol Lowering Atherosclerosis Study(CLAS),20) which was conducted on patients with established coronary artery disease, the relative risk for non-fatal myocardial infarction or coronary death was increased 2.1 times and the relative risk for any coronary event was increased 3.1 times. This study is the only study until now to has shown the prediction of risk with the progression of IMT and not with using the baseline value.20)
In addition to the IMT, high-frequency ultrasound (more than 7 MHz) can easily detect the presence of a plaque. However, there are no definitive criteria of atherosclerotic plaque; generally, the ultrasound definition of plaque is a focal thickening of the arterial wall intruding into the arterial lumen and having maximal thickness >1.3-1.5 mm(Fig. 1B). However, its measurement has a main limitation due to the necessity to express the result dichotomously, by the presence or absence of plaque, because precise quantitative measurement of a plaque's dimensions via ultrasound is not yet possible. One early prospective study in middle-aged healthy Finnish men has shown that the presence of carotid plaque multiplied the short-term incidence of acute myocardial infarction by about three, and the relative risk increased up to about seven times if plaque that caused >20% stenosis was present.7) A recent prospective populationbased cohort study on asymptomatic elderly people found that the number of plaques in the extra-cranial carotid arteries was associated with an increased age-adjusted risk of cardiovascular mortality in the year to come, by about 1.2-fold per 1 µm increase.9) In hypertensive populations, using carotid plaque to predict the CVD risk has also been prospectively shown.10)11) Carotid plaque is a powerful predictor of stroke or myocardial infarction.
Measuring the carotid IMT is a safe, noninvasive and relatively inexpensive method to detect the early changes of atherosclerosis. However, there are several limitations to apply this to patients. The measuring method hasn't yet been standardized, but this limitation will be overcome by the improving imaging technique of ultrasound. Further, the normal range, as regard to age and gender, must be defined more clearly. Thus, this method can be applied as one of the established series of test to identify and track the progression of atherosclerotic disease.
This technique has been widely used in Western countries for screening of asymptomatic individual who have multiple risk factors or they are under suspicion for harboring coronary artery disease. Coronary calcification is a marker of coronary atherosclerosis within the coronary vasculature, and this can be detected and quantified by electron beam computed tomography(EBCT)(Fig. 2).21) A high deposit of coronary calcium cannot indicate the site of a specific vulnerable lesion. However, this finding does suggest the probability of a vulnerable plaque.21) Thus, some advanced lesions may exist in the absence of calcium, and these lesions exist in the early phase of atherosclerosis and acute events are related with soft plaque. A few prospective studies have shown that a coronary calcium deposit may predict future cardiovascular events in asymptomatic subjects,22-24) but there is still debate as to whether the presence of coronary calcifications adds prognostic information to that provided by assessing the traditional risk factors. One study showed that the coronary calcium score was predictive of hard and soft coronary events in both gender.23) Yet another prospective study has shown that coronary calcifications do not provide incremental prognostic information to the Framingham coronary risk assessment of high-risk asymptomatic subjects.24)
More recently, after the introduction of multislice CT (MSCT), coronary atherosclerotic lesion can be seen directly.25-27) This method can non-invasively detect arterial stenosis like a coronary angiography, as well being able to evaluate the nature of plaque, like intravascular ultrasound can(Fig. 3). Therefore, for the high risk group, this method can be a promising test for evaluating the presence of coronary obstruction and to predict coronary heart disease more consistently than any other method. However there are several limitations to be solved, such as large amount of radiation exposure, the difficulty in cases of arrhythmia and the patients with impaired renal function and thyroid dysfunction, and the obese patients. In the near future, many studies will illuminate the prognostic value of this technique.
Endothelial dysfunction is known as one of the earliest signs of atherosclerosis. Brachial artery flow-mediated dilation(FMD) is a promising marker of endothelial function, and it can be measured by determining the dilatation of the brachial artery due to the release of nitric oxide by a transient high blood flow that's induced by a few minutes of forearm ischemia.28)29) FMD is currently considered as a valuable surrogate marker of nitric oxide release.
Ultrasound measurement of brachial artery reactivity is currently a well established and validated method to non-invasively detect endothelial dysfunction.30) FMD was assessed by measuring, with ultrasound unit electronic calipers, the change in the brachial artery diameter after 60 seconds of reactive hyperemia, as compared with the baseline measurements after the deflation of a cuff placed around the forearm that had been inflated to 50 mm Hg greater than the systolic blood pressure or to 250 mmHg for 5 minutes.30) High-frequency ultrasound measures the extent of flow-mediated vasodilation after hyperemia; the shear-mediated NO release causes brachial arterial vasodilation and the magnitude of the dilating response is representative of the endothelial function.31)32) It means that a healthy endothelium release more nitric oxide than does diseased endothelium (Fig. 4). There is debate on several aspect of the technique, as regards to upper arm or forearm cuff occlusion, and the occlusion pressure. The upper arm occlusion produces a greater percent change in arterial diameter than does forearm occlusion, and this is caused by higher shear stress or the direct effect of ischemia on the brachial artery.33) The change of arterial diameter after upper arm occlusion may be influenced by a metabolic effect or a myogenic source of the vasodilatory response. Therefore, the change of arterial diameter via upper arm occlusion results in overall dilatation that may not be entirely flow mediated or endothelial dependent. The duration of cuff inflation also affects the arterial diameter change, and the diameter increases continuously from 30 seconds to 5 minutes. However the change in arterial diameter is similar after 5 to 10 minutes of occlusion. The baseline arterial diameter also influences the percent change of the diameter. The percent change in the arterial diameter decreases as the baseline vessel diameter increases. Thus, an arterial diameter between 2.5 to 5 mm is recommended when performing the flow-mediated vasodilation test. The initial reaction time after the post-ischemic period was proposed as a new parameter of endothelial dysfunction.34) This parameter is more accurate than the conventional method to detect endothelial dysfunction, but this method needs validation.
Clinical outcome studies have demonstrated the relevance of endothelial dysfunction for predicting the future cardiovascular risk. Neunteafl et al.35) reported that those patients with impaired FMD(<10%) had a greater rate of revascularization procedures than did those patients with a preserved FMD(>10%) in a study on 73 patients with angina, and with 5 years follow up. Gokce et al.36) reported a similar result that brachial FMD is an independent predictor for assessing the first 30 days post-operative adverse events. However, there is insufficient evidence to support the FMD as a predictor of cardiovascular events. A recent study on participants in the Framingham study has shown that the FMD was related with many cardiovascular risk factors, suggesting that the FMD may be an integrator of the effects of cardiovascular risk factors on the arterial wall.37) Moreover, several studies have suggested that the brachial FMD may have prognostic value in identifying those subjects at risk for developing cardiovascular disease,28)35)38)39) but all these studies have had a relatively small sample size and it is unknown whether this prognostic information is independent of, or additional to, that provided by the traditional cardiovascular risk factors.
Although the FMD test, as a surrogate marker of early atherosclerosis, strongly demonstrates that endothelial function can predicts the future outcome, it is necessary to conduct a study with a large population and long-term data as to the FMD test's prognostic value in an intermediate risk or low risk group, and this must also be determined for a normal range of people, according to age and sex difference.
The definition of arterial stiffness is the material property of the arterial wall or the capacitive function of the vessel as a whole.41) The arterial system is composed of two systems that have different structure. Namely, the elastic arteries such as aorta, carotid and brachiocephalic artery, and they mainly serve a conduit and reserve function during the cardiac cycle. The second system is the muscular arteries that are more distal, and they have an active vasodilating effect on medication. The ability of arteries to accommodate the volume depends on the viscoelastic properties of the arterial wall, which is described in terms of compliance(C), distensibility(D), or stiffness. Compliance is defined as the change in volume (ΔV) due to a change in pressure(ΔP), C=ΔV/ΔP, and D=C=ΔV/ΔPV, where V is the baseline volume. Stiffness is the reciprocal value of distensibility.42) These parameters are dependent on the blood pressure, so the arteries become stiffer at high pressure. Ejection of blood into the aorta generates a pressure wave that is propagated to the other arteries throughout the body. This forward wave is reflected back by any part of the arterial tree, generating reflected waves that travel backward toward the aorta. Incident waves and reflected waves interact constantly, and the amplitude and shape of the blood pressure is determined by their timing. In low stiffening arteries, the reflected wave impacts on the ascending aorta during diastole, so the reflected wave has no influence on systolic BP, and this cause an elevation of diastolic pressure, resulting in a boosting effect on coronary circulation. In high stiffening arteries, the reflected waves impacts on the aorta during systole, increasing the systolic pressure and myocardial oxygen consumption, and decreasing the diastolic pressure and coronary perfusion. Arterial compliance is reduced in such pre-atherosclerotic disease as aging, hypertension, renal failure and diabetes.42) Therefore, measurement of arterial stiffness can be utilized to predict early atherosclerotic changes.
Several methods have recently been developed for measuring arterial stiffness. These measurements can be performed in superficial arteries, including the brachial, carotid, femoral and radial arteries, via such techniques such as ultrasound, mechanography and tonometry, and often with the assistance of computerized image analysis systems such as the echo tracking system.43)44) The stiffening component of large artery disease can be detected by measuring arterial wall motion(distension) or pulse wave velocity(generally between carotid and femoral sites) or by quantitative analysis of pulse wave contour(wave reflections). Among them, the measurement of pulse wave velocity(PWV) and the augmentation index(AI) may have potential for diagnostic application.46-49)
Alterations in the function and structure of the arterial wall are some of the earliest changes of aging and atherosclerosis, the same as endothelial dysfunction. However, measurement of arterial stiffness provides quantitative information regarding the status of the elastic properties of the arterial system, and arterial stiffening is not necessarily a sign of atherosclerosis; it may also represent the effects of hypertension and/or aging on the large artery walls.43) Although a large number of physiological, pharmacological and therapeutic studies have used arterial stiffening measures, there is not yet sufficient, concordant evidence that arterial stiffening might be a predictor of coronary and cardiovascular events. A few studies of special groups of very old subjects or of patients with end-stage renal disease have suggested that the aortic pulse wave velocity predicts fatal cardiovascular events.50)51) By contrast, a recent study in asymptomatic elderly men is inconsistent with the latter studies; it showed that stiffness of the carotid artery lacked additive prognostic value, and this was contrary to carotid artery plaques burden.9) These preliminary observations need further validation in larger and less specific populations with longer follow-up durations. More recently, the Conduit Artery Function Evaluation(CAFE) study, which is a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial(ASCOT), speculated that the central blood pressure transferred from the radial arterial pressure wave might explain the differential effects of blood pressure-lowering drugs on the cardiovascular structure and the clinical outcomes in other recent outcome trials.52-54)
The main purposes for detecting subclinical arterial disease are to identify asymptomatic subjects, to provide additional lifestyle changes or drug therapies and to better monitor the effects of therapy. Improved detection of asymptomatic high CVD risk individuals is needed for better targeting of intensive risk-reduction treatment and optimizing the cost-effectiveness of primary prevention. Detection of high CVD risk is still based on traditional risk factor assessment in standard clinical practice. However, non-invasive imaging methods have limitations for accurately diagnosing early atherosclerosis(Table 2). and this may add substantially to the prognostic information obtained with using the traditional risk factors for clinically silent subjects. Improvement of imaging technique and the appearance of new imaging modalities may increase the usefulness of imaging tests for making the diagnosis of early atherosclerosis.
Figures and Tables
 | Fig. 1Carotid intima-media thickness and plague. A: automated computerized measure of carotid intima-media thickness. B: atherosclerotic plaque in right common carotid artery (arrow). |
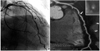 | Fig. 3Multislice CT (right) and coronary angiographic view of LAD artery. Arrow indicate insignificant stenosis of mid LAD artery in angiography, but MSCT shows significant plaque burden of same site. CT: computed tomography, LAD: left anterior descending, MSCT: multislice CT. |
 | Fig. 4Mechanism of endothelial-dependant and endothelial-independent vasodilation. GTP: guanosine triphosphate, cGMP: cyclic guanosine monophosphate. |
References
1. Kuulasmaa K, Tunstall-Pedoe H, Dobson A, et al. Estimation of contribution of changes in classic risk factors to trends in coronary. Lancet. 2000. 355:675–687.
2. Fletcher GF, Balady GJ, Vogel RA. Preventive cardiology: how can we do better? Proceedings of the 33rd Bethesda Conference; 2001 Dec 18; Bethesda, Maryland: USA. J Am Coll Cardiol. 2002. 40:580–651.
3. Smith SC Jr, Greenland P, Grundy SM. Prevention conference V: beyond secondary prevention: identifying the high-risk patient for primary prevention: executive summary. Circulation. 2000. 101:111–116.
4. Greenland P, Smith SC Jr, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation. 2001. 104:1863–1867.
5. Popma JJ. Braunwald's Heart Disease. 2005. 7th ed. 423.
6. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imagimg. Circulation. 1986. 74:1399–1406.
7. Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb. 1991. 11:1245–1249.
8. Hollander M, Bots ML, Del Sol AI, et al. Carotid plaque increases the risk of stroke and subgroups of cerebral infarction in asymptomatic elderly: the Rotterdam Study. Circulation. 2002. 105:2872–2877.
9. Stork S, van den Beld AW, von Schacky C, et al. Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population-based cohort study. Circulation. 2004. 110:344–348.
10. Zanchetti A, Rosei EA, Dal Palu C, Leonetti G, Magnani B, Pessina A. The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long-term randomized treatment with either verapamil or chlorthalidone on carotid intima-media thickness. J Hypertens. 1998. 16:1667–1676.
11. Zanchetti A, Bond MG, Hennig M, et al. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation. 2002. 106:2422–2427.
12. Hyun DW, Kim KS, Hur SH. Correlation between the carotid intima-media thickness and the plaque burden of the left main coronary artery using ultrasonography. Korean Circ J. 2005. 35:795–800.
13. Simon A, Gariepy J, Chironi G, Megnien JL, Levenson J. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002. 20:159–169.
14. Simon A, Gariepy J, Moyse D, Levenson J. Differential effects of nifedipine and co-amilozide on the progression of early carotid wall changes. Circulation. 2001. 103:2949–2954.
15. Hyun DW, Bae JH, Kim KY, Hwang IK, Kim WS. Measurement of the carotid intima, media and intima-media thickness with ultrasound and new software. Korean Circ J. 2005. 35:625–632.
16. Choi YS, Youn HJ, Hong EJ, et al. The prediction of high echogenicity of intimal area in carotid artery for the plaque burden of culprit lesion in coronary artery disease. Korean Circ J. 2006. 36:458–464.
17. Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997. 146:483–494.
18. Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997. 96:1432–1437.
19. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999. 340:14–22.
20. Hodis HN, Mack WJ, LaBree L, et al. The role of intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998. 128:262–269.
21. O'Rourke RA, Brundage BH, Froelicher VF, et al. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation. 2000. 102:126–140.
22. Arad Y, Spaduro LA, Goodman K, et al. Predictive value of electron beam computed tomography of the coronary arteries: 19-month follow-up of 1173 asymptomatic subjects. Circulation. 1996. 93:1951–1953.
23. Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003. 107:2571–2576.
24. Detrano RC, Wong ND, Doherty TM, et al. Coronary calcium does not accurately predict near-tern future coronary events in high-risk adults. Circulation. 1999. 99:2633–2638.
25. Schoepf UJ, Becker CR, Ohnesorge BM, Yucel EK. CT of coronary artery disease. Radiology. 2004. 232:18–37.
26. de Feyter PJ, Nieman K. Noninvasive multi-slice computed tomography coronary angiography: an emerging clinical modality. J Am Coll Cardiol. 2004. 44:1238–1240.
27. Schoenhagen P, Hallibruton SS, Stillman AE, et al. Noninvasive imaging of coronary arteries: current and future role of multidetector row CT. Radiology. 2004. 232:7–17.
28. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002. 39:257–265.
29. Levenson J, Pessana F, Gariepy J, Armentano R, Simon A. Gender differences in wall shear-mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol. 2001. 38:1668–1674.
30. Celemajor DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992. 340:1111–1115.
31. Gordon JB, Ganz P, Nabel EG, et al. Atherosclerosis influences the vasomotion responses of epicardial coronary artery to exercise. J Clin Invest. 1989. 83:1946–1952.
32. Zeiher AM, Drexler H, Wollschlaeger H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol. 1989. 14:1181–1190.
33. Berry KL, Skyrme-Jones RA, Meredith IT. Occlusion cuff position is an important determinant of the time and magnitude humanbrachial artery flow-mediated dilation. Clin Sci. 2000. 99:261–267.
34. Lee SJ, Lee DW, Kim KS, Lee IK. Comparison of the lag time to initiation of flow-mediated vasodilation to endothelium-dependent vasodilation in the early diagnosis of endothelial dysfunction. Korean Circ J. 2001. 31:867–876.
35. Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated vasodilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000. 86:207–210.
36. Gokce N, Keaney JF Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003. 41:1769–1775.
37. Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation. Hypertension. 2004. 44:134–139.
38. Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow mediated-dilation to ankle-brachial pressure index. Circulation. 2003. 108:2093–2098.
39. Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol. 2003. 42:1037–1043.
40. Wattanakit K, Folsom AR, Selvin E, et al. Risk factors for peripheral arterial disease incidence in persons with diabetes. Atherosclerosis. 2005. 180:389–397.
41. Asmar RG. Arterial Stiffness and Pulse Wave Velocity: clinical applications. 1999. Paris: Elsevier.
42. van der Heijden-Spek JJ, Staessens JA, Fagard RH, Hoeks P, Boudier HA, van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension. 2000. 35:637–642.
43. Simon A, Levenson J. Effect of hypertension on viscoelasticity of large arteries in humans. Curr Hypertens Rep. 2001. 3:74–79.
44. Graf S, Gariepy J, Massonneau M, et al. Experimental and clinical validation of arterial diameter waveform and intimal media thickness obtained from B-mode ultrasound image processing. Ultrasound Med Biol. 1999. 25:1353–1363.
45. Vlachopoulos C, Hirata K, O'Rourke MF. Pressure-altering agents affect central aortic pressures more than is apparent from upper limb measurements. In hypertensive patients: the role of arterial wave reflections. Hypertension. 2001. 38:1456–1460.
46. European Society of Hypertension-European Society of Cardiology Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003. 21:1011–1053.
47. Lee YS, Kim KS, Nam CW, et al. Clinical implication of carotid-radial pulse wave velocity for patients with coronary artery disease. Korean Circ J. 2006. 36:565–572.
48. Lee YS, Kim KS, Nam CW, Kim YN. Increased arterial stiffness in patients with cardiac syndrome X: pulse wave velocity in cardiac syndrome X. Korean Circ J. 2005. 35:424–428.
49. Rhee MY, Han SK, Lyu S, Lee MY, Kim YK, Yu SM. Short-term treatment with angiotensin II antagonist in essential hypertension: effects of losartan on left ventricular diastolic function, left ventricular mass, and aortic stiffness. Korean Circ J. 2000. 30:1341–1349.
50. Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001. 21:2046–2050.
51. Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998. 32:570–574.
52. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006. 113:1213–1225.
53. De Buyzere LM, Segers P. Peripheral or central augmentation index: an esoteric question or a non-invasive clue to central haemodynamics? J Hypertens. 2007. 25:289–293.
54. Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001. 38:922–926.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download