REFERENCES
1. DeLellis RA. Pathology and genetics of tumours of endocrine organs. World Health Organization classification of tumours;Lyon: IARC Press;2004. p. 147–150.
2. Spoudeas HA. Paediatric endocrine tumours. West Sussex UK: Novo Nordisk;2005. p. 81–92.
3. Waguespack SG, Rich T, Grubbs E, Ying AK, Perrier ND, Ayala-Ramirez M, Jimenez C. A current review of the etiology, diagnosis, and treatment of pediatric pheochromocytoma and paraganglioma. Journal of Clinical Endocrinology & Metabolism. 95(5):2023–2037. 2010.

4. Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, Giordano TJ, Greene LA, Goldstein DS, Lehnert H, Manger WM, Maris JM, Neumann HP, Pacak K, Shulkin BL, Smith DI, Tischler AS, Young WF Jr. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 11(3):423–36. 2004.

5. Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer research. 63(17):5615–5621. 2003.
6. Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 26(5):551–66. 2002.
7. Strong VE, Kennedy T, Al-Ahmadie H, Tang L, Coleman J, Fong Y, Brennan M, Ghossein RA. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery. 143(6):759–68. 2008.

8. de Krijger RR, van der Harst E, van der Ham F, Stijnen T, Dinjens WN, Koper JW, Bruining HA, Lamberts SW, Bosman FT. Prognostic value of p53, bcl-2, and c-erbB-2 protein expression in phaeochromocytomas. The Journal of pathology. 188(1):51–55. 1999.

9. August C, August K, Schroeder S, Bahn H, Hinze R, Baba HA, Kersting C, Buerger H. CGH and CD 44/MIB-1 immunohistochemistry are helpful to distinguish metastasized from nonmeta-stasized sporadic pheochromocytomas. Modern pathology. 17(9):1119–1128. 2004.

10. Lam KY, Lo CY, Wat NM, Luk JM, Lam KS. The clinicopathological features and importance of p53, Rb, and mdm2 expression in phaeochromocytomas and paragangliomas. Journal of clinical pathology. 54(6):443–448. 2001.

11. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. The Journal of Immunology. 133(4):1710–1715. 1984.
12. Nagura S, Katoh R, Kawaoi A, Kobayashi M, Obara T, Omata K. Immunohistochemical estimations of growth activity to predict biological behavior of pheochromocytomas. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 12(12):1107. 1999.
13. Elder EE, Xu D, Höög A, Enberg U, Hou M, Pisa P, Gruber A, Larsson C, Bäckdahl M. KI-67 and hTERT expression can aid in the distinction between malignant and benign pheochromocytoma and paraganglioma. Modern pathology. 16(3):246–255. 2003.

14. Pinato DJ, Ramachandran R, Toussi STK, Vergine M, Ngo N, Sharma R, Lloyd T, Meeran K, Palazzo F, Martin N, Khoo B, Dina R, Tan TM. Immunohistochemical markers of the hypoxic response can identify malignancy in phaeochromocytomas and paragangliomas and optimize the detection of tumours with VHL germline mutations. British Journal of Cancer. 2013; 108:429–437.

15. Stojadinovic A, Ghossein RA, Hoos A, Nissan A, Marshall D, Dudas M, Cordon-Cardo C, Jaques DP, Brennan MF. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. Journal of Clinical Oncology. 20(4):941–950. 2002.

16. Pham TH, Moir C, Thompson GB, Zarroug AE, Hamner CE, Farley D, van Heerden J, Lteif AN, Young WF Jr. Pheochromocytoma and paraganglioma in children: a review of medical and surgical management at a tertiary care center. Pediatrics. 118(3):1109–1117. 2006.

17. Burnichon N, Rohmer V, Amar L, Herman P, Leboulleux S, Darrouzet V, Niccoli P, Gaillard D, Chabrier G, Chabolle F, Coupier I, Thieblot P, Lecomte P, Bertherat J, Wion-Barbot N, Murat A, Venisse A, Plouin PF, Jeunemaitre X, Gimenez-Roqueplo AP. PGL.NET network. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab. 94(8):2817–2827. 2009.

18. McNicol AM. Differential diagnosis of pheochromocytomas and paragangliomas. Endocrine pathology. 12(4):407–415. 2001.

19. Manger WM, Eisenhofer G. Pheochromocytoma: diagnosis and management update. Current hypertension reports. 6(6):477–484. 2004.

20. Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan A, Patel S, Waguespack S, Jimenez C. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 96(3):717–725. 2011.

21. Tischler AS. Pheochromocytoma and extra-adrenal paraganglioma: updates. Arch Pathol Lab Med. 132(8):1272–1284. 2008.

22. Wu D, Tischler AS, Lloyd RV, DeLellis RA, de Krijger R, van Nederveen F, Nosé V. Observer variation in the application of the Pheochromocytoma of the Adrenal Gland Scaled Score. The American journal of surgical pathology. 33(4):599–608. 2009.

23. Pollard PJ, El-Bahrawy M, Poulsom R, Elia G, Killick P, Kelly G, Hunt T, Jeffery R, Seedhar P, Barwell J, Latif F, Gleeson MJ, Hodgson SV, Stamp GW, Tomlinson IP, Maher ER. Expression of HIF-1α, HIF-2α (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. Journal of Clinical Endocrinology & Metabolism. 91(11):4593–4598. 2006.

24. Ein SH, Pullerits J, Creighton R, Balfe JW. Pediatric pheochromocytoma. A 36-year review. Pediatr Surg Int. 12(8):595–598. 1997.

25. Perel Y, Schlumberger M, Marguerite G, Alos N, Revillon Y, Sommelet D, De Lumley L, Flamant F, Dyon JF, Lutz P, Heloury H, Lemerle J. Pheochromocytoma and paraganglioma in children: a report of 24 cases of the French Society of Pediatric Oncology. Pediatr Hematol Oncol. 14(5):413–422. 1997.

26. Reddy VS, O'Neill JA Jr, Ho GW 3rd, Neblett WW 3rd, Pietsch JB, Morgan WM 3rd, Goldstein RE. Twenty-five-year surgical experience with pheochromocytoma in children. Am Surg. 66(12):1085–1092. 2000.
Table 1.
Immunohistochemical Panel
| Antibody | Primary antibody∗ | Company | Dilution | Buffer(pH)† | Target Stain‡ | Cutoff Values§ |
|---|---|---|---|---|---|---|
| Ki-67 | mm | DAKO, Carpinteria, California, USA | 1:200 | CA (6) | N | > 2 % |
| p53 | mm | DAKO | 1:200 | CA (6) | N | > 5 % |
| mdm-2 | mm | Leica Biosystems, Heidelberg, Germany | 1:100 | EDTA (9) | N | > 50 % |
| Santa Cruz | ||||||
| p21 | mm | biotechnology, Dallas, | 1:200 | CA (6) | N | > 10 % |
| Texas, USA | ||||||
| Spring Bioscience, | ||||||
| p27 | rp | Pleasaton, California, | 1:300 | CA (6) | N | > 30 % |
| USA | ||||||
| bcl-2 | mm | DAKO | 1:100 | CA (6) | C | > 50 % |
| cyclin D1 | rp | Santa Cruz biotechnology | 1:100 | CA (6) | N | > 5 % |
Table 2.
Clinical Information of the Patients with PHEO and aPGL
| Total (n=20) | PHEO (n=14) | aPGL (n=6) | P value | |
|---|---|---|---|---|
| Age (months, mean±SD) | 143.90 ±40.87 | 141.93 ±39.26 | 148.50 ±19.60 | .775∗ |
| Gender (M : F) | 15 : 5 | 9 : 5 | 6 : 0 | .260† |
| Location | .829† | |||
| Right (%) | 7 (35) | 5 (35.7) | 2 (33.3%) | |
| Left (%) | 6 (30) | 5 (35.7) | 1 (16.7%) | |
| Bilateral (%) | 7 (35) | 4 (28.6) | 3 (50.0%) | |
| Greatest dimension (cm, mean±SD) | 4.86 ±1.50 | 4.84 ±1.26 | 4.88 ±2.08 | .966† |
| Malignancy (%) | 7 (35) | 4 (28.6) | 3 (50.0%) | .613† |
| Hereditary Features (%) | 4 (20) | 4 (28.6) | 0 | .267† |
| Chemotherapy (%) | 2 (10) | 0 | 2 (33.3%) | .079† |
| Radiotherapy (%) | 6 (30) | 3 (21.4) | 3 (50.0%) | .303† |
| Follow-up(months, mean±SD) | 97.25 ±55.67 | 95.64 ±37.03 | 101.00 ±90.47 | .850∗ |
| Disease free survival (months, mean±SD) | 82.20 ±58.25 | 82.43 ±43.86 | 81.67 ±88.82 | .985∗ |
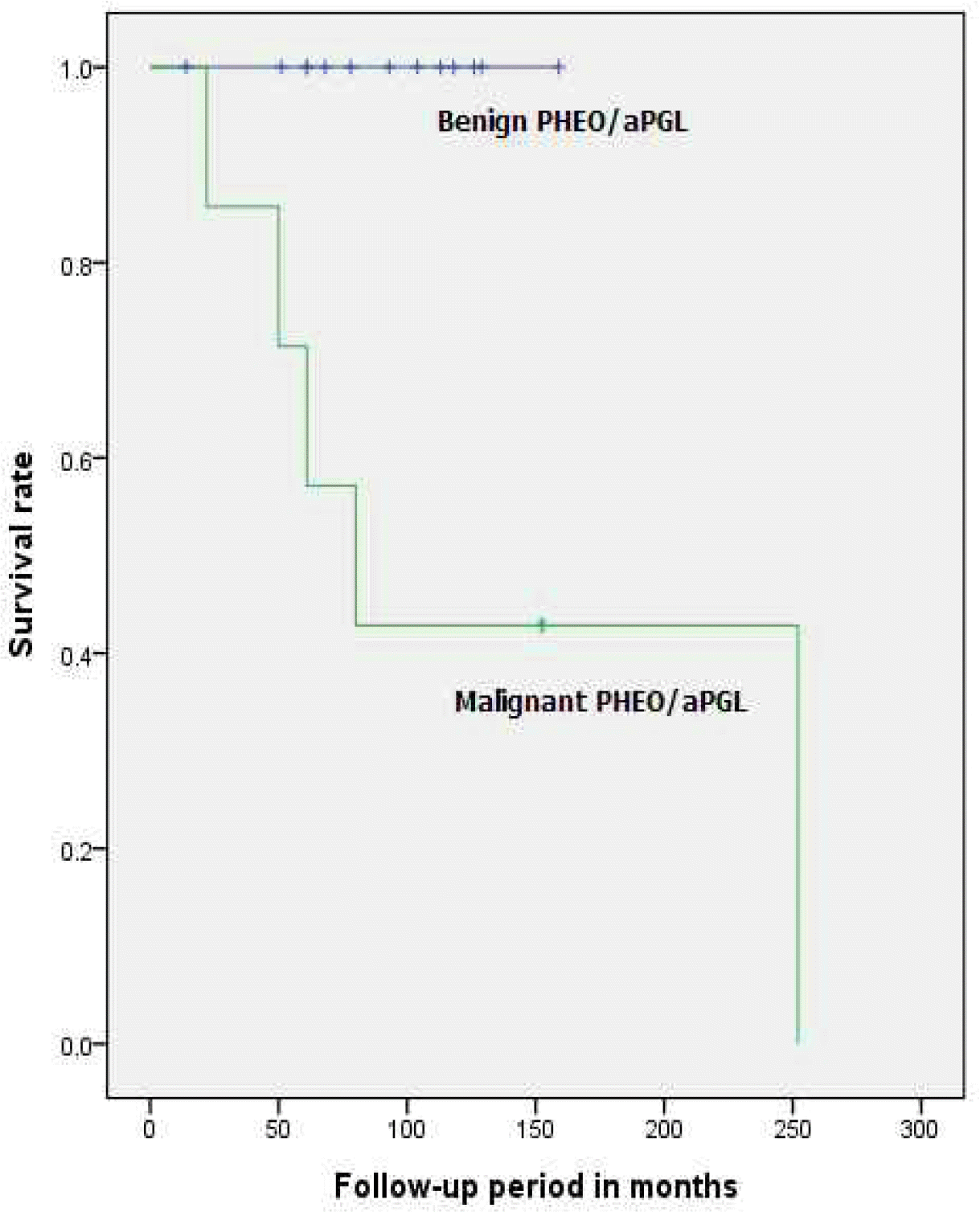
| Median survival (months, range) | 86.20 (14-252) | 86.50 (50-159) | 82.50 (14-252) | NA§ |
Table 3.
Clinical Information of the Patients with Benign and Malignant Tumors∗
| Benign (n=13) | Malignant (n=7) | P value | |
|---|---|---|---|
| Age (months, mean±SD) | 143.77 ± 46.25 | 144.14 ± 31.78 | .983† |
| Gender (M : F) | 10 : 3 | 5 : 2 | > .999‡ |
| Hereditary Features (%) | 3 (23.1) | 1 (14.3) | > .999‡ |
| Chemotherapy (%) | 0 | 2 (28.6) | .111‡ |
| Radiotherapy (%) | 0 | 6 (85.7) | < .001‡ |
| Location | .381‡ | ||
| Right (%) | 5 (38.5) | 2 (28.6) | |
| Left (%) | 5 (38.5) | 1 (14.3) | |
| Bilateral (%) | 3 (23.1) | 4 (57.1) | |
| Greatest dimension (cm, mean±SD) | 5.01 ± 1.55 | 4.57 ± 1.44 | .547† |
| Clinical manifestation | |||
| Hypertension (%) | 10 (76.9) | 4 (57.1) | .613‡ |
| Palpitation (%) | 7 (53.8) | 3 (42.9) | > .999‡ |
| Headache (%) | 6 (46.2) | 2 (28.6) | .642‡ |
| Diaphoresis (%) | 7 (53.8) | 2 (28.6) | .374‡ |
| Flushing (%) | 9 (69.2) | 2 (28.6) | .160‡ |
| Nausea, vomiting (%) | 7 (53.8) | 3 (42.9) | > .999‡ |
| Follow-up (months, mean±SD) | 90.38 ± 39.48 | 110.00 ± 80.02 | .467† |
| Disease free survival (months, mean±SD) | 90.38 ± 39.48 | 67.00 ± 84.92 | .690† |
| Median survival period (months, range) | 93.00 (14-159) | 80.00 (22-252) | NA§ |
Table 4.
Microscopic Features of Benign and Malignant Tumors∗
| Benign (n=13) | Malignancy (n=7) | P value† | |
|---|---|---|---|
| Capsular invasion (n, %) | 4 (30.8) | 3 (42.9) | .651 |
| Vascular invasion (n, %) | 0 | 4 (57.1) | .007 |
| Extension into adipose tissue (n, %) | 1 (7.69) | 4 (57.1) | .031 |
| Presence of large nests (n, %) | 7 (53.8) | 1 (14.3) | .158 |
| Central tumor necrosis (n, %) | 6 (46.2) | 5 (71.4) | >.999 |
| High cellularity (n, %) | 3 (23.1) | 2 (28.6) | >.999 |
| Tumor cell spindling (n, %) | 8 (61.5) | 1 (14.3) | .070 |
| Cellular monotony (n, %) | 3 (23.1) | 1 (14.3) | >.999 |
| Mitosis (n, %) | 1 (7.69) | 4 (57.1) | .031 |
| Atypical mitosis (n, %) | 1 (7.69) | 2 (28.6) | .270 |
| Nuclear pleomorphism (n, %) | 4 (30.8) | 0 | .249 |
| Nuclear hyperchromasia (n, %) | 9 (69.2) | 2 (28.6) | .160 |
Table 5.
Microscopic Features of Benign and Malignant PHEO∗
| Benign (n=10) | Malignancy (n=4) | P value† | |
|---|---|---|---|
| Capsular invasion (n, %) | 3 (30) | 2 (50) | .580 |
| Vascular invasion (n, %) | 0 | 3 (75) | .033 |
| Extension into adipose tissue (n, %) | 0 | 3 (75) | .003 |
| Presence of large nests (n, %) | 7 (70) | 0 | .023 |
| Central tumor necrosis (n, %) | 5 (50) | 4 (100) | .089 |
| High cellularity (n, %) | 3 (30) | 1 (25) | .857 |
| Tumor cell spindling (n, %) | 7 (70) | 1 (25) | .139 |
| Cellular monotony (n, %) | 3 (30) | 0 | .234 |
| Mitosis (n, %) | 1 (10) | 3 (75) | .019 |
| Atypical mitosis (n, %) | 0 | 1 (25) | .114 |
| Nuclear pleomorphism (n, %) | 3 (30) | 0 | .234 |
| Nuclear hyperchromasia (n, %) | 8 (80) | 0 | .008 |
Table 6.
Analysis of PASS Score and Pathologic Score between Benign and Malignant Tumor∗
| Benign (n=13) | Malignancy (n=7) | P value | |
|---|---|---|---|
| PASS Score (mean±SD) | 6.00 ± 2.86 | 7.57 ± 3.36 | .317† |
| PASS ≥ 4 (n, %) | 10 (76.9) | 5 (71.4) | >.999‡ |
| PASS ≥ 6 (n, %) | 8 (61.5) | 5 (71.4) | >.999‡ |
| Pathologic Score (mean±SD) | 0.31 ± 0.86 | 3.43 ± 2.57 | .001† |
| Score < 2 (n, %) | 12 (92.3) | 2 (28.5) | .007§ |
| Score ≥ 2 (n, %) | 1 (7.7) | 5 (71.4) |
Table 7.
Immunohistochemistry∗
| Antibody | Benign (n=13) | Malignancy (n=7) | P value† |
|---|---|---|---|
| Ki-67 (n, %) | 2 (15.4) | 4 (57.1) | .122 |
| p53 (n, %) | 2 (15.4) | 3 (42.9) | .290 |
| bcl-2 (n, %) | 8 (61.5) | 4 (57.1) | >.999 |
| mdm-2 (n, %) | 2 (15.4) | 3 (42.9) | .290 |
| cyclin D1 (n, %) | 0 | 0 | >.999 |
| p21 (n, %) | 3 (23.1) | 3 (42.9) | .613 |
| p27 (n, %) | 2 (15.4) | 3 (42.9) | .290 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download