Abstract
Purpose
To investigate the relationship between placental pathology and neurodevelopmental outcomes among extremely low birth weight (ELBW) infants
Methods
Pathology of placentas from ELBW infants born at a tertiary neonatal intensive care unit from January 2007 to December 2012 were reviewed and placental histology was grouped into 3 categories by a designated pathologist: acute chorioamnionitis (ACA), maternal vascular underperfusion (MVU), and control group. Matched ELBW infants were tested for significant neurodevelopmental delays defined as mental developmental index (MDI) or psychomotor developmental index (PDI) <70, using Bayley Scales of Infant Development-II (BSID-II).
Results
The mean gestational age and birth weight of 175 infants were 27.1±2.5 weeks and 764.7±152.3 g respectively. Placental histology revealed MVU (48.0%), ACA (25.1%) and control (26.9%) in distribution. There were less significant patent ductus arteriosus in MVU group than in control group [adjusted odds ratio (OR)=0.331, P=0.011]. The frequencies of other neonatal diseases and mortality were similar in 3 groups. Sixty four of 175 infants were examined for BSID-II at mean corrected 19.9±3.2 months. MVU was associated with significant mental developmental delay (OR=5.185, P=0.036), but after adjustment for head circumference/weight at birth, the statistically significance of association disappeared (adjusted OR=4.391, P=0.075). ACA did not affect neonatal and neurodevelopmental outcomes.
References
1. Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation–a workshop report. Placenta. 2005; 26(Suppl A):S114–7.
2. Perrone S, Toti P, Toti MS, Badii S, Becucci E, Gatti MG, et al. Perinatal outcome and placental histological characteristics: a single-center study. J Matern Fetal Neonatal Med. 2012; 25(Suppl 1):110–3.

3. Dammann O, Brinkhaus MJ, Bartels DB, Dordelmann M, Dressler F, Kerk J, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev. 2009; 85:325–9.

4. Hartling L, Liang YY, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and metaanalysis. Arch Dis Child Fetal Neonatal Ed. 2012; 97:F8–17.

5. Mitra S, Aune D, Speer CP, Saugstad OD. Chorioamnionitis as a Risk Factor for Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. Neonatology. 2014; 105:189–99.

6. Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: A metaanalysis. JAMA. 2000; 284:1417–24.

7. Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003; 290:2677–84.

8. Suppiej A, Franzoi M, Vedovato S, Marucco A, Chiarelli S, Zanardo V. Neurodevelopmental outcome in preterm histological chorioamnionitis. Early Hum Dev. 2009; 85:187–9.

9. Rovira N, Alarcon A, Iriondo M, Ibanez M, Poo P, Cusi V, et al. Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants. Early Human Dev. 2011; 87:253–7.

10. Andrews WW CS, Biasini F, Peralta-Carcelen AM, Rector R, Alriksson-Schmidt Al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008; 198:446.e1–11.
11. Mu SC, Lin CH, Sung TC, Chen YL, Lin YC, Lee CC, et al. Neurodevelopmental outcome of very-low-birthweight infants with chorioamnionitis. Acta paediatr Taiwan. 2007; 48:207–12.
12. Polam S, Koons A, Anwar M, Shen-Schwarz S, Hegyi T. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Pediatr Adolesc Med. 2005; 159:1032–5.

13. Oqunyemi D MM, Jackson U, Hunter N, Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med. 2003; 13:102–9.
14. van Vliet EOG, de Kieviet JF, van der Voorn JP, Been JV, Oosterlaan J, van Elburg RM. Placental pathology and longterm neurodevelopment of very preterm infants. Am J Obstet Gynecol. 2012; 206:489.e1–7.

15. Parra-Saavedra M CF, Triunfo S, Savchev S, Pequero A, Nadal A, et al. Neurodevelopmental outcome of near-term small-for-gestational-age infants with and without signs of placental underperfusion. Placenta. 2014; 35:269–74.
16. Son SH, Choi KY, Lee JM, Shin SH, Kim C, Kim YJ, et al. Comparison of the incidences of neonatal morbidities by different criteria of histologic chorioamnionitis in extremely low gestational age newborns. Neontal Med. 2013; 20:35–41.

17. Yoo C, Jang DG, Jo YS, Yoo J, Lee G. Pathologic differences between placentas from intrauterine growth restriction pregnancies with and without absent or reveresed end diastolic velocity of umbilical arteries Korean J Pathol. 2011; 45:36–44.
18. Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC pediatr. 2003; 3:13.

19. Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013; 45:446–54.

20. Bell MJ TJ, Feigin RD, Keating JP, Marshall R, Barton L. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978; 187:1–7.
21. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–34.

22. Jobe AH, Ikegami M. Prevention of bronchopulmonary dysplasia. Curr Opin Pediatr. 2001; 13:124–9.

23. Almog B, Shehata F, Aljabri S, Levin I, Shalom-Paz E, Shrim A. Placenta weight percentile curves for singleton and twins deliveries. Placenta. 2011; 32:58–62.

24. Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev. 2002; 60:S19–25.

25. Kovo M, Schreiber L, Ben-Haroush A, Wand S, Golan A, Bar J. Placental vascular lesion differences in pregnancy-induced hypertension and normotensive fetal growth restriction. Am J Obstet Gynecol. 2010; 202:561.e1–5.

26. Aviram R, T BS, Kidron D. Placental aetiologies of foetal growth restriction: clinical and pathological differences. Early Hum Dev. 2010; 86:59–63.

27. Vedmedovska N, Rezeberga D, Teibe U, Melderis I, Donders GG. Placental pathology in fetal growth restriction. Eur J Obst Gynecol Reprod Biol. 2011; 155:36–40.

28. Rakza T, Magnenant E, Klosowski S, Tourneux P, Bachiri A, Storme L. Early hemodynamic consequences of patent ductus arteriosus in preterm infants with intrauterine growth restriction. J Pediatr. 2007; 151:624–8.

29. Been JV, Lievense S, Zimmermann LJI, Kramer BW, Wolfs TGAM. Chorioamnionitis as a risk factor for necrotizing enterocolitis: A systematic review and metaanalysis. J Pediatr. 2013; 162:236–42.e2.
30. Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002; 22:106–32.

31. Savchev S, Sanz-Cortes M, Cruz-Martinez R, Arranz A, Botet F, Gratacos E, et al. Neurodevelopmental outcome of fullterm small-for-gestational-age infants with normal placental function. Ultrasound Obstet Gynecol. 2013; 42:201–6.

32. Gavilanes AW, Strackx E, Kramer BW, Gantert M, Van den Hove D, Steinbusch H, et al. Chorioamnionitis induced by intraamniotic lipopolysaccharide resulted in an interval-dependent increase in central nervous system injury in the fetal sheep. Am J Obstet Gynecol. 2009; 200:437.e1–8.

33. Feldhaus B, Dietzel ID, Heumann R, Berger R. Effects of interferon-gamma and tumor necrosis factor-alpha on survival and differentiation of oligodendrocyte progenitors. J Soc Gynecol Investig. 2004; 11:89–96.
34. Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012; 67:287–94.

35. Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Nurosci. 2006; 26:4752–62.

36. Redline R, Minich N, Taylor HG, Hack M. Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg). Pediatr Dev Pathol. 2007; 10:282–92.
37. Nasef N, Shabaan AE, Schurr P, Iaboni D, Choudhury J, Church P, et al. Effect of clinical and histological chorioamnionitis on the outcome of preterm infants. Am J Perinatol. 2013; 30:59–68.

38. Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. Eur J Neurosci. 2001; 13:1101–6.

Fig. 1.
Light microscopic findings of placental lesions. (A)-(C) compatible with amniotic fluid infection. (A) Umbilical vasculitis, a classical fetal inflammatory response (H-E stain, x100). (B) Necrotizing funisitis showing diffuse band of neutrophil debris in the Wharton's jelly (X40). (C) Acute chorioamnionitis of the chorioamniotic membranes, indicative of maternal inflammatory response. There is diffuse infiltration of the maternal neutrophils into the amnion (X200). Figure D-F compatible with maternal vascular underperfusion. (D) Increased syncytial knots. Almost every chorionic villus has syncytial knots (X100). (E) Acute villous infarct showing coagulation necrosis (X100). (F) Increased intervillous fibrin (X100).
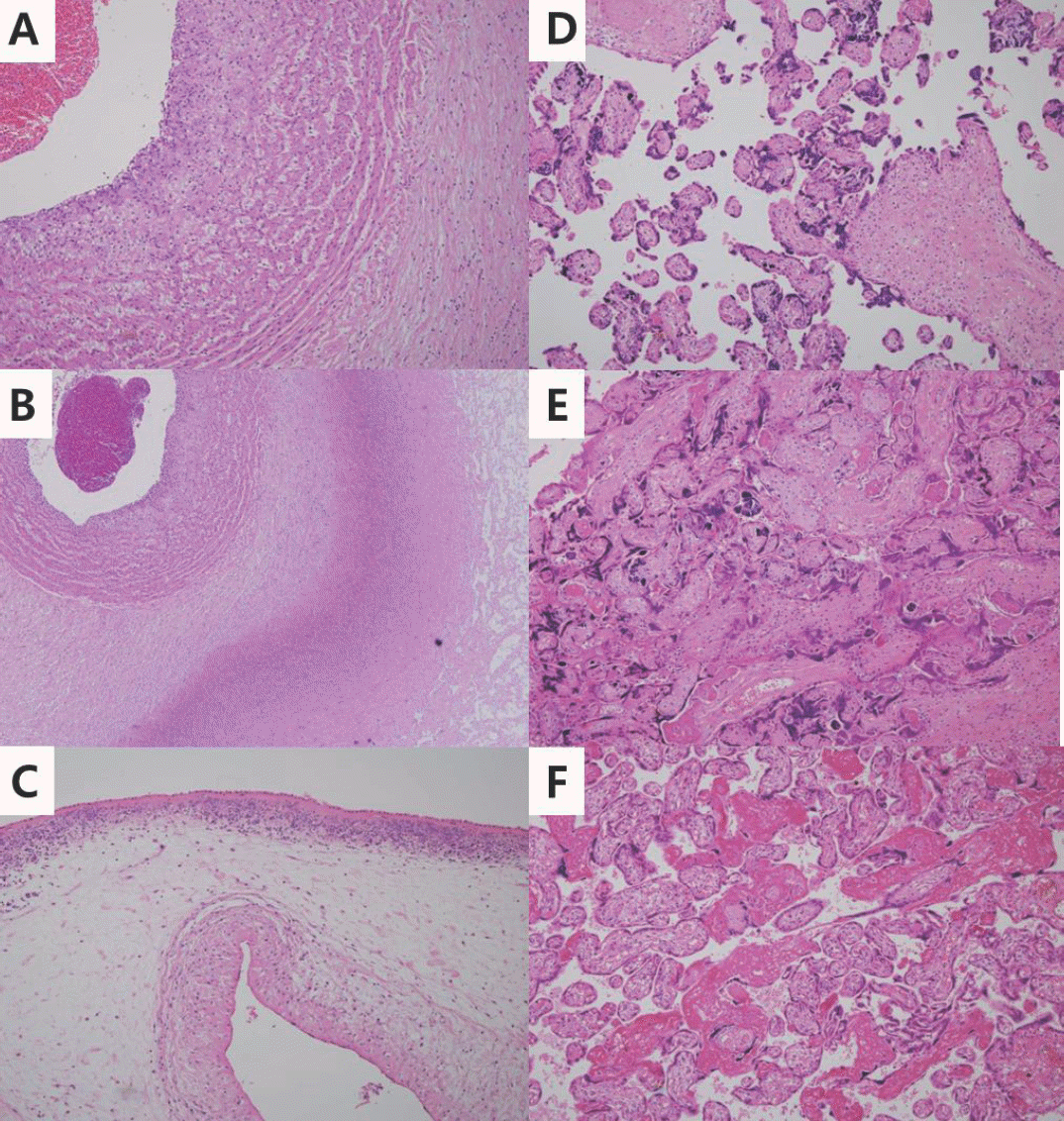
Table 1.
Perinatal characteristics
| Characteristics | Control (n=47) | ACA (n=44) | MVU (n=84) | P-value |
|---|---|---|---|---|
| Gestational age (weeks)∗ | 26.4±2.3 | 25.6±1.2 | 28.2±2.6 | <0.001 |
| Birth weight (g)∗ | 766.5±139.2 | 816.9±121.0 | 736.4±167.4 | 0.035 |
| SGA, n (%) | 13 (26.5) | 0 (0) | 51 (60.7) | <0.001 |
| HC (cm)∗ | 23.8±1.5 | 23.6±1.8 | 24.3±2.0 | 0.025 |
| HC <10p, n (%) | 12 (25.0) | 4 (9.5) | 44 (53.0) | <0.001 |
| HC/weight (cm/g X100)∗ | 3.2±0.5 | 2.9±0.3 | 3.4±0.8 | <0.001 |
| Male, n (%) | 23 (48.9) | 21 (47.7) | 52 (61.9) | 0.211 |
| Multiple gestation, n (%) | 31 (63.3) | 17 (38.6) | 30 (35.7) | 0.002 |
| Apgar score at 1min∗ | 3.5±1.7 | 3.2±1.5 | 3.9±2.0 | 0.099 |
| Apgar score at 5mins∗ | 5.8±1.5 | 5.4±1.4 | 6.3±1.8 | 0.002 |
| Initial pH∗ | 7.2±0.1 | 7.2±0.1 | 7.2±0.1 | 0.325 |
| Initial base deficit (mmEq/L)∗ | 6.0±4.4 | 7.3±5.0 | 6.8±5.0 | 0.462 |
| Lactic acid (mmol/L)∗ | 4.6±2.9 | 4.3±2.8 | 5.8±3.7 | 0.064 |
| Cesarean delivery, n (%) | 28 (57.1) | 14 (31.8) | 72 (85.7) | <0.001 |
| PPROM > 18hrs, n (%) | 7 (14.3) | 17 (38.6) | 9 (10.7) | <0.001 |
| Oligohydramnios | 5 (10.6) | 1 (2.3) | 13 (15.5) | 0.079 |
| PIH, n (%) | 2 (4.1) | 1 (2.3) | 28 (33.3) | <0.001 |
| Gestational diabetes, n (%) | 2 (4.3) | 0 (0) | 2 (2.4) | 0.474 |
| Antenatal corticosteroid, n (%) | 27 (55.1) | 25 (56.8) | 53 (63.1) | 0.789 |
| Maternal age (years)∗ | 31.0±4.2 | 33.3±3.3 | 31.5±4.2 | 0.017 |
| Maternal education (College) | 37 (78.7) | 36 (81.8) | 61 (72.6) | 0.473 |
| Placental weight (g)∗,† | 211.7±69.5 | 249.6±92.6 | 203.6±79.9 | 0.011 |
| Placental weight<10p, n (%)† | 8 (53.3) | 10 (37.0) | 51 (80.9) | <0.001 |
| Birth weight/placental weight (g/g)∗,† | 3.9±1.4 | 3.6±1.0 | 4.2±1.3 | 0.170 |
Table 2.
Perinatal mortality and morbidity among neonates with acute chorioamnionitis and maternal vascular underperfusion
| ACA | MVU | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% | % CI | P-value∗ | Odds ratio | 95% | % CI | P-value∗ | |
| Mortality | 1.150 | 0.379 | 3.491 | 0.805 | 2.139 | 0.741 | 6.173 | 0.160 |
| PDA | 0.468 | 0.190 | 1.151 | 0.098 | 0.331 | 0.141 | 0.778 | 0.011 |
| NEC | 0.648 | 0.234 | 1.789 | 0.402 | 0.598 | 0.204 | 1.750 | 0.348 |
| Early sepsis | 1.421 | 0.218 | 9.285 | 0.714 | 2.257 | 0.359 | 14.202 | 0.386 |
| Late sepsis | 0.962 | 0.385 | 2.403 | 0.934 | 0.561 | 0.235 | 1.341 | 0.193 |
| IVH | 0.715 | 0.234 | 2.185 | 0.556 | 0.861 | 0.284 | 2.605 | 0.791 |
| PVL | 2.430 | 0.549 | 10.753 | 0.242 | 0.944 | 0.168 | 5.312 | 0.948 |
| ROP | 1.916 | 0.579 | 6.334 | 0.287 | 1.688 | 0.478 | 5.965 | 0.416 |
| BPD | 0.382 | 0.129 | 1.131 | 0.082 | 0.801 | 0.297 | 2.159 | 0.662 |
Abbreviations: ACA, acute chorioamnionitis; MVU, maternal vascular underperfusion; CI, confidence interval; PDA, patent ductus arteriosus; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; BPD, bronchopulmonary dysplasia
Table 3.
Comparisons of neurodevelopmental delay based on the placental pathology
Table 4.
Neurodevelopmental outcome among neonates with acute chorioamnionitis or maternal vascular underperfusion
Table 5.
Neurodevelopmental outcome among neonates with acute chorioamnionitis or maternal vascular underperfusion after multivariable logistic regression
| Developmental index | Placental pathology | Odds ratio | 95% CI | P-value∗ |
|---|---|---|---|---|
| 70≤MDI<85 | Control | 1 | ||
| ACA | 1.931 | 0.325 11.476 | 0.469 | |
| MVU | 1.845 | 0.398 8.558 | 0.434 | |
| MDI<70 | Control | 1 | ||
| ACA | Infinite | 0.963 | ||
| MVU | 4.391 | 0.863 22.357 | 0.075 | |
| 70≤PDI<85 | Control | 1 | ||
| ACA | 0.619 | 0.082 4.660 | 0.641 | |
| MVU | 0.581 | 0.118 2.869 | 0.505 | |
| PDI<70 | Control | 1 | ||
| ACA | 0.533 | 0.075 3.788 | 0.529 | |
| MVU | 0.620 | 0.147 2.612 | 0.515 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download