Abstract
Purpose
Intrauterine inflammation (IUI) is a leading cause of preterm delivery. Although matrix metalloproteinase-8 (MMP-8) and intercellular adhesion molecule-1 (ICAM-1) are known to be related with IUI, it has not been fully elucidated whether MMP-9 or ICAM-3 is associated with IUI. We performed this study to determine whether the levels of tumor necrosis factor-alpha (TNF-α), MMP-9 and ICAM-3 in umbilical cord blood of preterm infants are associated with chorioamnionitis, funisitis or bronchopulmonary dysplasia.
Methods
Eighty-two pairs of pregnant women and their preterm newborns <35 weeks gestation were enrolled. Levels of TNF-α, MMP-9 and ICAM-3 in umbilical cord blood were measured using immunoassays and compared with results of histological examination of placenta and clinical data of the study participants. Results: The level of MMP-9 in umbilical cord blood was significantly associated with the presence of funisitis (P=0.007). The level of TNF-α in umbilical cord blood was significantly associated with the development of bronchopulmonary dysplasia (P=0.030). However, presence of chorioamnionitis or funisitis was not associated with development of bronchopulmonary dysplasia. With the establishment of receiver operating characteristic (ROC) curve, the best cut-off value for umbilical blood MMP-9 was 99.42 pg/mL in identification of funisitis. The area under a constructed ROC curve for prediction of funisitis was 0.847 (standard error, 0.112; 95% confidence interval, 0.750–0.917).
Conclusion
Measurement of MMP-9 concentration in umbilical cord blood may be an alternative way to predict whether a preterm infant has been exposed to IUI. Further study with larger numbers of subjects will be necessary to elucidate the association between the presence of IUI and neonatal adverse outcome.
Go to : 
References
1. Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intraamniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004; 191:1339–45.

3. Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997; 11:135–76.

4. Arntzen KJ, Kjollesdal AM, Halgunset J, Vatten L, Austgulen R. Tnf, il-1, il-6, il-8 and soluble tnf receptors in relation to chorioamnionitis and premature labor. J Perinat Med. 1998; 26:17–26.

5. Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (il-6), il-8 and granulocyte colony stimulating factor (g-csf) in term and preterm parturition. Cytokine. 1993; 5:81–8.

6. Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992; 166:1576–87.

7. Tanaka Y, Narahara H, Takai N, Yoshimatsu J, Anai T, Miyakawa I. Interleukin-1beta and interleukin-8 in cervicovaginal fluid during pregnancy. Am J Obstet Gynecol. 1998; 179:644–9.
8. Winkler M, Fischer DC, Hlubek M, van de Leur E, Haubeck HD, Rath W. Interleukin-1beta and interleukin-8 concentrations in the lower uterine segment during parturition at term. Obstet Gynecol. 1998; 91:945–9.

9. Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010; 37:339–54.

10. Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol. 2001; 32:623–9.

11. Kashlan F, Smulian J, Shen-Schwarz S, Anwar M, Hiatt M, Hegyi T. Umbilical vein interleukin 6 and tumor necrosis factor alpha plasma concentrations in the very preterm infant. Pediatr Infect Dis J. 2000; 19:238–43.

12. Andrys C, Drahosova M, Hornychova H, Tambor V, Musilova I, Tosner J, et al. Umbilical cord blood concentrations of il-6, il-8, and mmp-8 in pregnancy complicated by preterm premature rupture of the membranes and histological chorioamnionitis. Neuro Endocrinol Lett. 2010; 31:857–63.
13. Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996; 174:1433–40.

14. Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000; 183:1124–9.

15. Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989; 73:383–9.

17. Weiss A, Goldman S, Shalev E. The matrix metalloproteinases (mmps) in the decidua and fetal membranes. Front Biosci. 2007; 12:649–59.

18. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007; 25:21–39.

19. Park CW, Yoon BH, Park JS, Jun JK. A fetal and an intraamniotic inflammatory response is more severe in preterm labor than in preterm prom in the context of funisitis: Unexpected observation in human gestations. PLoS One. 2013; 8:e62521.

20. Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001; 185:1156–61.

21. Fukunaga S, Ichiyama T, Maeba S, Okuda M, Nakata M, Sugino N, et al. Mmp-9 and timp-1 in the cord blood of premature infants developing bpd. Pediatr Pulmonol. 2009; 44:267–72.

22. Oh KJ, Park KH, Kim SN, Jeong EH, Lee SY, Yoon HY. Predictive value of intraamniotic and serum markers for inflammatory lesions of preterm placenta. Placenta. 2011; 32:732–6.

23. Makrakis E, Grigoriou O, Kouskouni E, Vitoratos N, Salamalekis E, Chatzoudi E, et al. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in plasma/serum and urine of women during term and threatened preterm labor: A clinical approach. J Matern Fetal Neonatal Med. 2003; 14:170–6.

24. Koucky M, Germanova A, Kalousova M, Hill M, CindrovaDavies T, Parizek A, et al. Low maternal serum matrix metalloproteinase (mmp)-2 concentrations are associated with preterm labor and fetal inflammatory response. J Perinat Med. 2010; 38:589–96.

25. Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intraamniotic infection. Am J Obstet Gynecol. 2000; 183:887–94.

26. Leviton A, Hecht JL, Allred EN, Yamamoto H, Fichorova RN, Dammann O. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol. 2011; 90:235–43.

27. Curley AE, Sweet DG, Thornton CM, O'Hara MD, Chesshyre E, Pizzotti J, et al. Chorioamnionitis and increased neonatal lung lavage fluid matrix metalloproteinase-9 levels: Implications for antenatal origins of chronic lung disease. Am J Obstet Gynecol. 2003; 188:871–5.

28. Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, et al. Matrix metalloproteinases-2, −8, and −9 and timp-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics. 2001; 108:686–92.

29. Sweet DG, Curley AE, Chesshyre E, Pizzotti J, Wilbourn MS, Halliday HL, et al. The role of matrix metalloproteinases −9 and −2 in development of neonatal chronic lung disease. Acta Paediatr. 2004; 93:791–6.

30. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006; 195:1578–89.
31. Radi ZA, Kehrli ME, Jr. , Ackermann MR. Cell adhesion molecules, leukocyte trafficking, and strategies to reduce leukocyte infiltration. J Vet Intern Med. 2001; 15:516–29.

32. Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by il 1 and interferon-gamma: Tissue distribution, biochemistry, and function of a natural adherence molecule (icam-1). J Immunol. 1986; 137:245–54.
33. Bleijs DA, Binnerts ME, van Vliet SJ, Figdor CG, van Kooyk Y. Low-affinity lfa-1/icam-3 interactions augment lfa-1/icam-1-mediated t cell adhesion and signaling by redistribution of lfa-1. J Cell Sci. 2000; 113(Pt 3):391–400.

34. Steinborn A, Sohn C, Scharf A, Geka F, Heger S, Kaufmann M. Serum intercellular adhesion molecule-1 levels and histologic chorioamnionitis. Obstet Gynecol. 2000; 95:671–6.

35. D'Alquen D, Kramer BW, Seidenspinner S, Marx A, Berg D, Groneck P, et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005; 57:263–9.
36. Mercer BM, Crouse DT, Goldenberg RL, Miodovnik M, Mapp DC, Meis PJ, et al. The antibiotic treatment of pprom study: Systemic maternal and fetal markers and perinatal outcomes. Am J Obstet Gynecol. 2012; 206:145. e141–9.

Go to : 
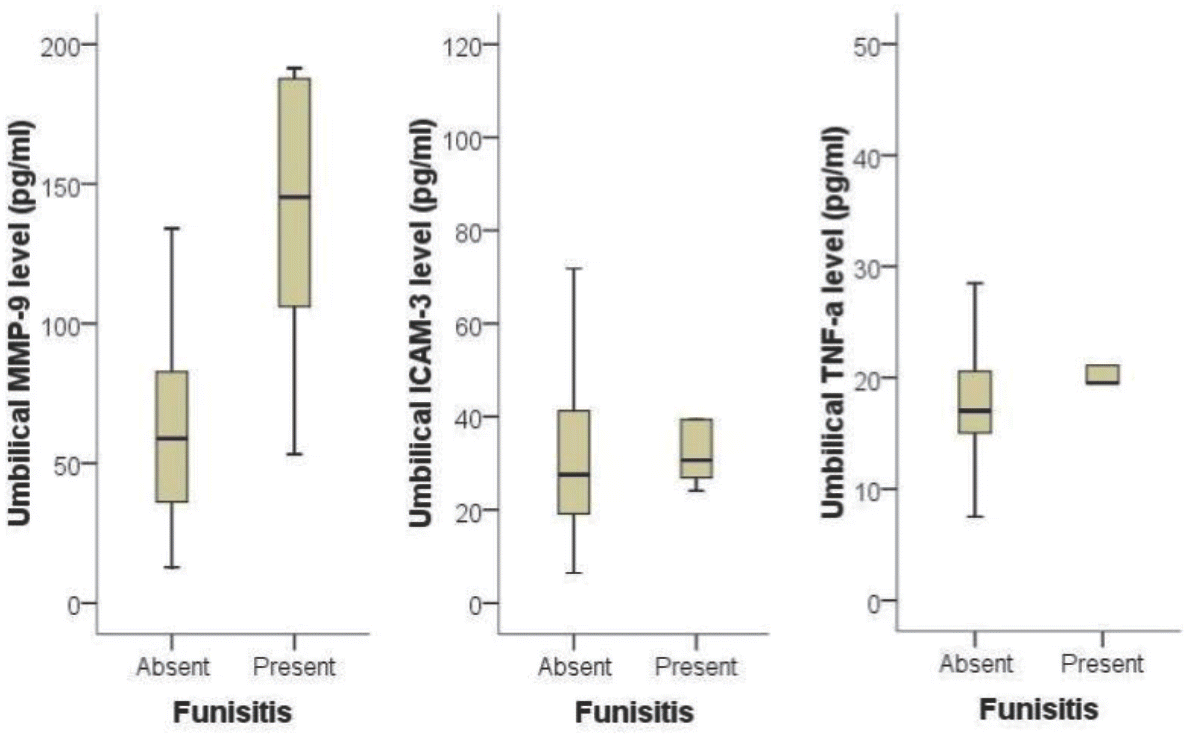 | Fig. 1.Comparison of matrix metalloproteinase-9 (MMP-9), intercellular adhesion molecule-3 (ICAM-3) and tumor necrosis factor-α (TNF-α) level in umbilical cord blood according to the presence or absence of funisitis. Infants from mother with funisitis had a significantly higher level of MMP-9 than those without funisitis (P=0.007). |
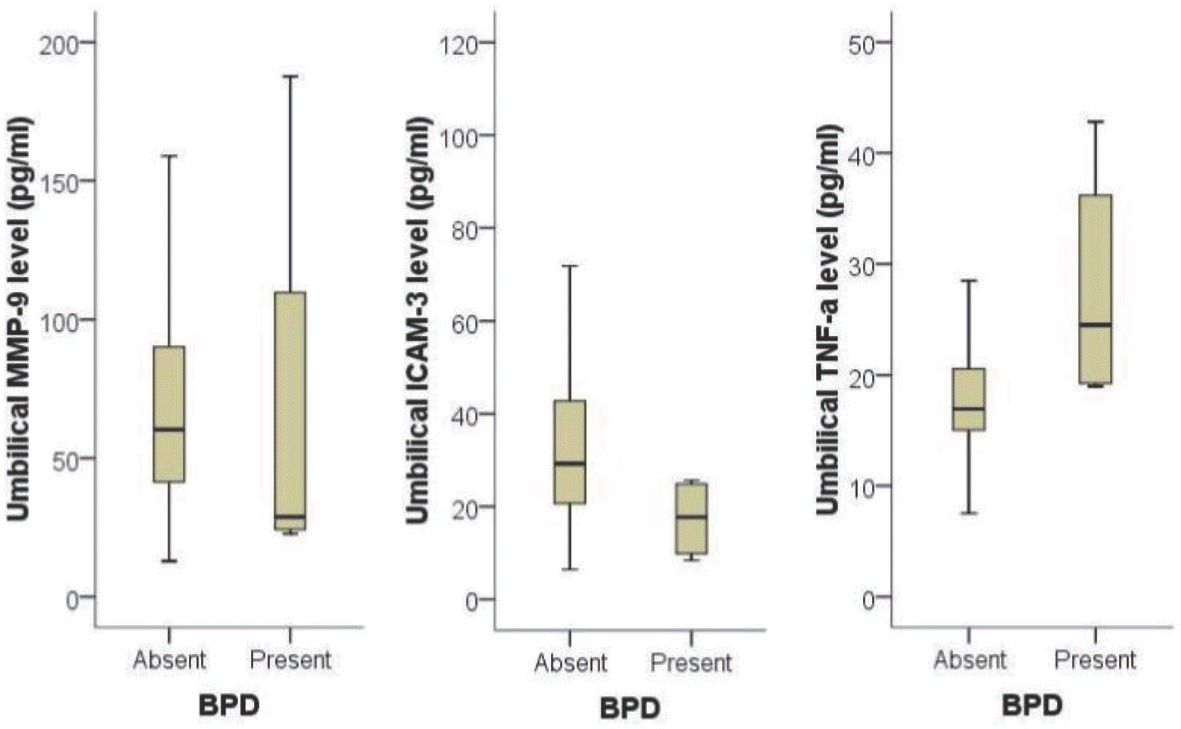 | Fig. 2.Comparison of matrix metalloproteinase-9 (MMP-9), intercellular adhesion molecule-3 (ICAM-3) and tumor necrosis factor-α (TNF- α) level in umbilical cord blood according to the presence or absence of bronchopulmonary dysplasia (BPD). Infants with BPD had a significantly higher level of TNF-α than those without funisitis (P=0.030). Although the median level of ICAM-3 in infants with BPD was lower than those without BPD, there was no statistical significance when the gestational age was adjusted as a cofounding factor. |
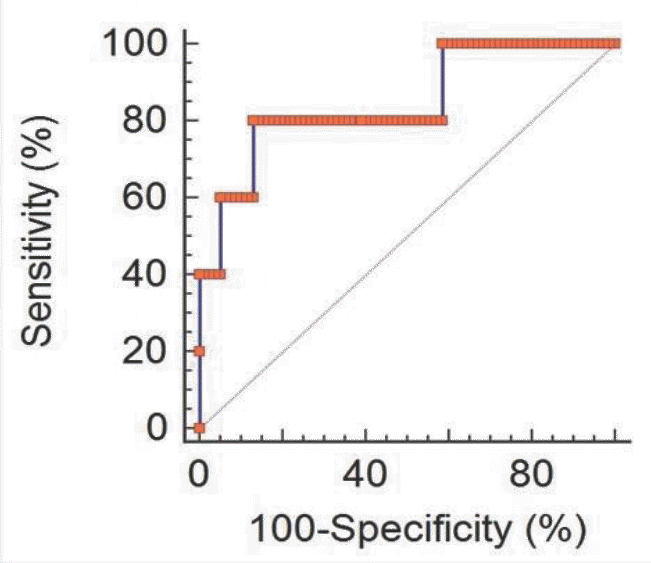 | Fig. 3.Receiver operating characteristic curves for identification of the presence of funisitis. Area under the curve for umbilical matrix metalloproteinase-9 level: 0.847 (95% confidence interval: 0.750–0.917, P=0.0019). |
Table 1.
Clinical characteristics of the study participants∗




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download