Abstract
Purpose
We investigated the effects of hypothyroidism on feeding advancement in very low birth weight infants (VLBW).
Methods
This study was a retrospective case-control study of 14 very low birth weight infants (VLBWIs) diagnosed with hypothyroidism and other 14 infants were recruited as age- and weight-matched controls without hypothyroidism or hypothyroxinemia in Seoul National University Children's Hospital between January 2007 and August 2009. We examined whether these infants gained weight more, achieved full-volume enteral feedings sooner, had fewer episodes of increased pre-gavage residuals, and had fewer days of parenteral nutrition.
Results
Until full enteral feeding (120 mL/kg/day) was not statistically significant between the groups. In the hypothyroidism group, during the first 14 days after birth, the volume of feeding was smaller [14.7 (0.5–84.0) mL/ kg/day, P=0.041], the episodes of increased pre-gavage residuals were frequently observed [16.7 (0.2–78) times, P=0.036], and the duration of central line was significantly longer [18 (10–50) days, P=0.018]. In hypothyroidism group, mean day at first L-thyroxine supplementation was 24.2±10.2 days after birth. L-thyroxine administration boosted thyroid function for hypothyroidism infants, helped them tolerate a larger amount of enteral feeding [from 89.5 (2.9–160.8) to 146.9 (31.8–178.8) mL/kg/day, P=0.002] and decreased episodes of excessive gastric residuals [from 5.5 (0–41.6) to 0 (0–44) time, P=0.026]. However, no more weight gain was statistically found.
References
1. Frank JE, Faix JE, Hermos RJ, Mullaney DM, Rojan DA, Mitchell ML, et al. Thyroid function in very low birth weight infants: effects on neonatal hypothyroidism screening. J Pediatr. 1996; 128:548–54.

2. Hong KB, Park JY, Chang YP, Yu J. Thyroid dysfunction in premature infants. Korean J Pediatr. 2009; 52:991–8.

3. Kim YC, Seo HJ, Lee KH, Ko CW, Kim HM. Thyroid dysfuction in premature infants. J Korean Soc Neonatol. 2005; 12:165–71.
4. Simic N, Asztalos EV, Rovet J. Impact of neonatal thyroid hormone insufficiency and medical morbidity on infant neurodevelopment and attention following preterm birth. Thyroid. 2009; 19:395–401.

7. Jadcherla SR, Kliegman RM. Studies of feeding intolerance in very low birth weight infants: definition and significance. Pediatrics. 2002; 109:516–7.

8. Komiyama M, Takahashi N, Yada Y, Koike Y, Honma Y, Aihara T, et al. Hypothyroxinemia and effectiveness of thyroxin supplementation in very low birth weight infants with abdominal distension and poor weight gain. Early Hum Dev. 2009; 85:267–70.

9. Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967; 71:159–63.

10. Charafeddine L, D'Angio CT, Phelps DL. Atypical chronic lung disease patterns in neonates. Pediatrics. 1999; 103:759–65.

12. Yaylali O, Kirac S, Yilmaz M, Akin F, Yuksel D, Demirkan N, et al. Dose Hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009; 2009:529802.
14. Shafer RB, Prentiss RA, Bond JH. Gastrointestinal transit in thyroid disease. Gastroenterology. 1984; 86:852–5.
15. Lucas A, Bloom SR, Aynsley-Green A. Postatal surge in plasma gut hormones in term and preterm infants. Biol Neonate. 1982; 41:63–7.
16. Valdez MG, Go VLW, Berseth CL. Gestational maturation of regionally distributed gastrointestinal hormones in preterm and term infants. Pediatr Res. 1990; 27:55A.
17. Berseth CL. Effect of early feeding on maturation of the preterm infant's small intestine. J Pediatr. 1992; 120:947–53.

18. Sagara K, Shimada T, Fujiyama S, Sato T. Serum gastrin levels in patients with thyroid dysfunction. Gastroenterol Jpn. 1983; 18:79–83.

19. Seino Y, Matsukura S, Inoue Y, Kadowaki S, Mori K, Imura H. Hypogastrinemia in hypothyroidism. Am J Dig Dis. 1978; 23:189–91.

20. Sellappan B, Chakraborty M, Cherian S. Congenital hypothyroidism presenting as pseudoobstruction in preterm infants. BMJ Case Rep. In press. 2014.
Fig. 1.
Comparison of feeding volumes (A) and episodes of residues per day during 14 days after birth (B) between the hypothyroidism group and control group.
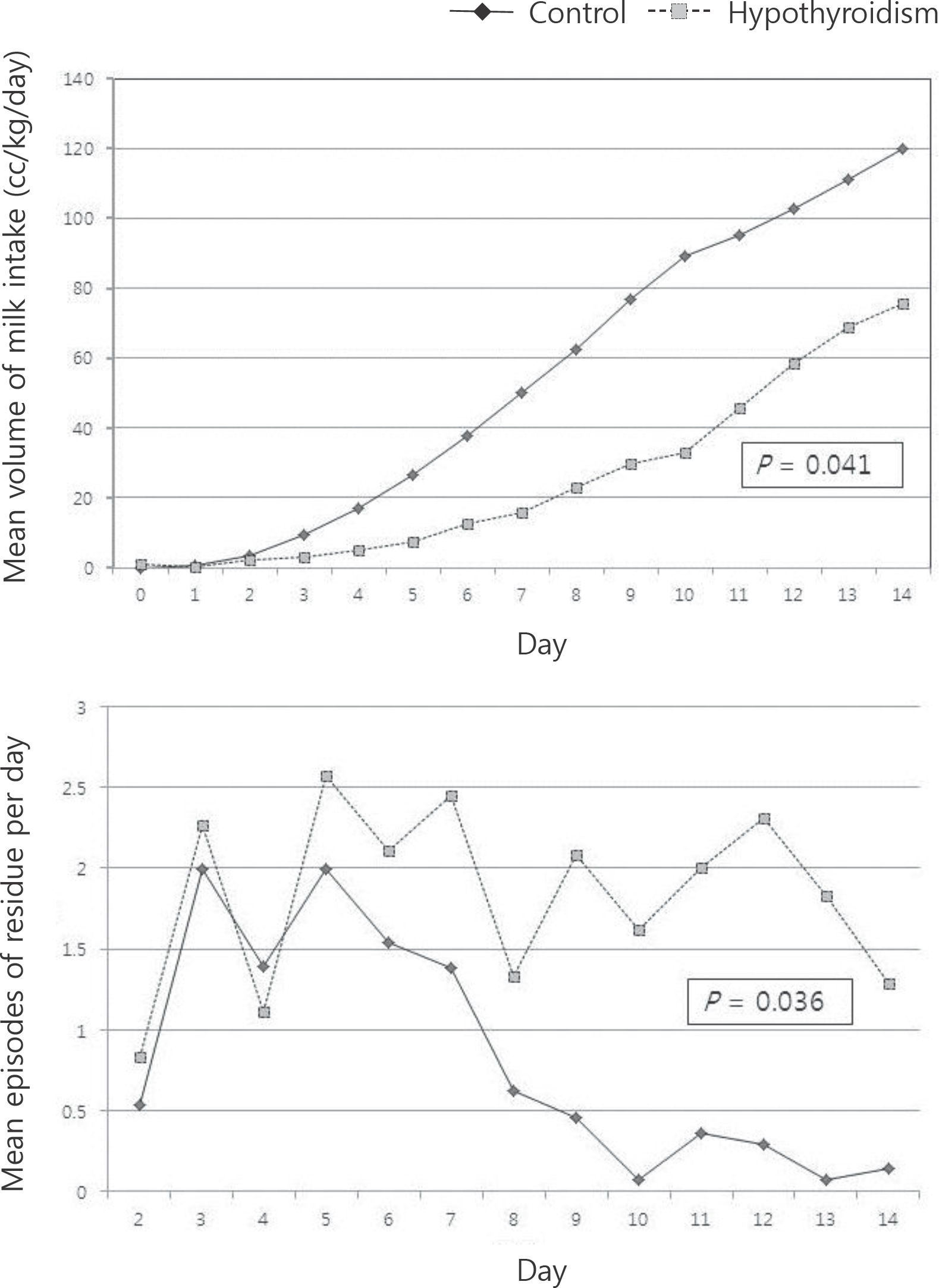
Fig. 2.
In hypothyroidism infants, comparison of episodes of gastric residues per day between 2 weeks before and after L-thyroxine administration, respectively. They showed fewer episodes of residue per day after L-thyroxine administration (P=0.01). In control group, “day 0” means the 24 days after birth which is the mean day of L-thyroxine administration for hypothyroidism infants.
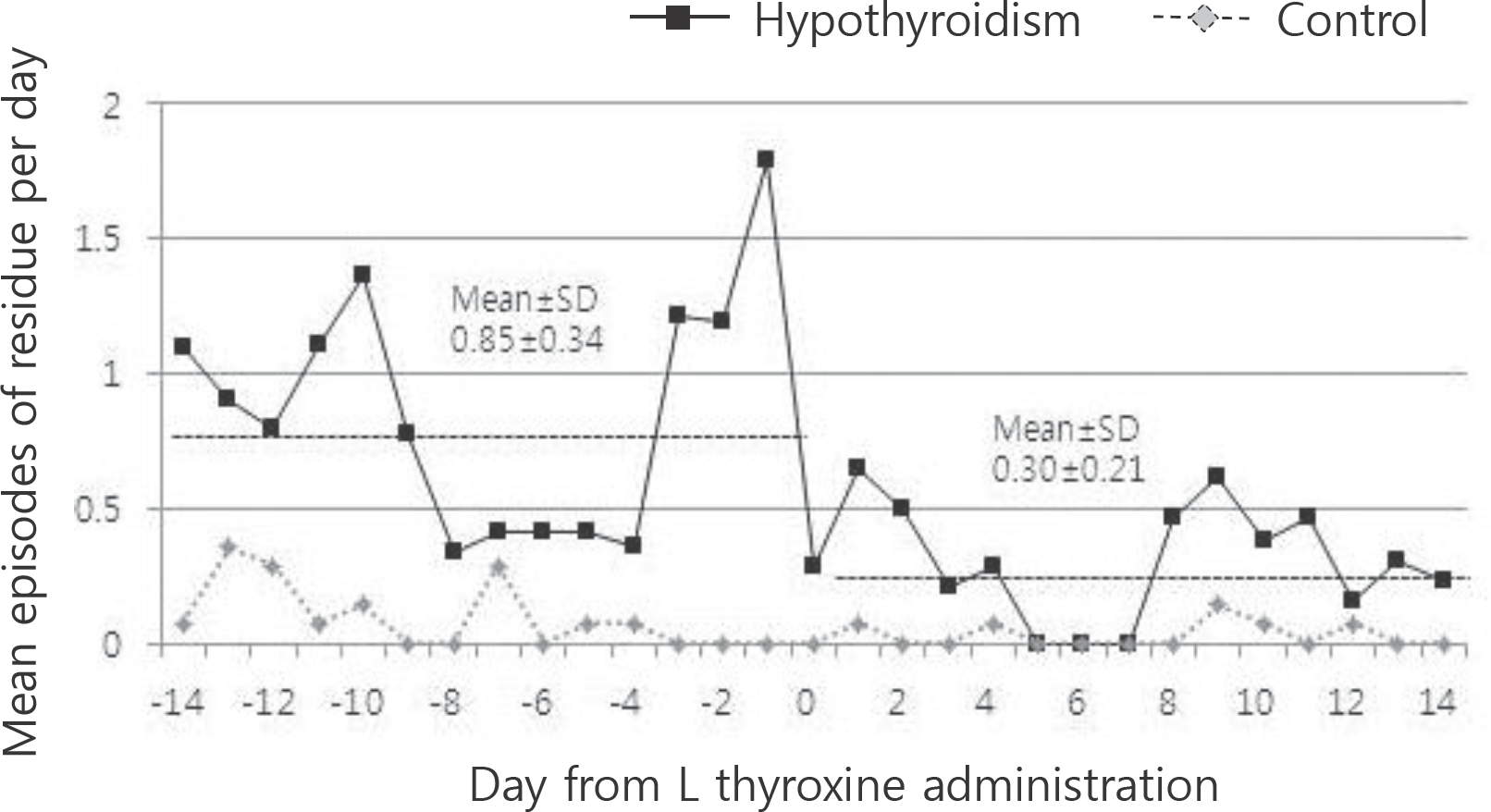
Table 1.
Demographics and first thyroid function test in study population
Table 2.
Comparison of weight gain and feeding tolerance during the first 14 days of life between hypothyroidism group and control group
Table 3.
Comparison of thyroid function, weight gain and feeding tolerance between 2 weeks before and after L-thyroxine administration in hypothyroidism group (n=14)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download