Abstract
Purpose
This study was performed to investigate the oxidation and antioxidation capacity in the maternal venous plasma of preterm labor with intact membranes, and premature rupture of membranes (PPROM), and also to evaluate their roles in the pathophysiology of PPROM.
Methods
Seventy five women in the following categories had venous blood retrieved: (1) Group A, normal pregnancy (n=24). (2) Group B, preterm labor with intact membranes (n=25). (3) Group C, preterm premature rupture of membranes (n=26). Malondialdehyde (MDA) levels as a marker of lipid peroxidation by thiobarbituric acid reaction, protein carbonyl content by 2,4-dinitrophenylhydrazine reaction, and total antioxidant capacity by oxygen radical absorbance capacity assay (ORAC) were measured. Mann-Whitney U test, Kruskal-Wallis test were used for statistical analysis.
Results
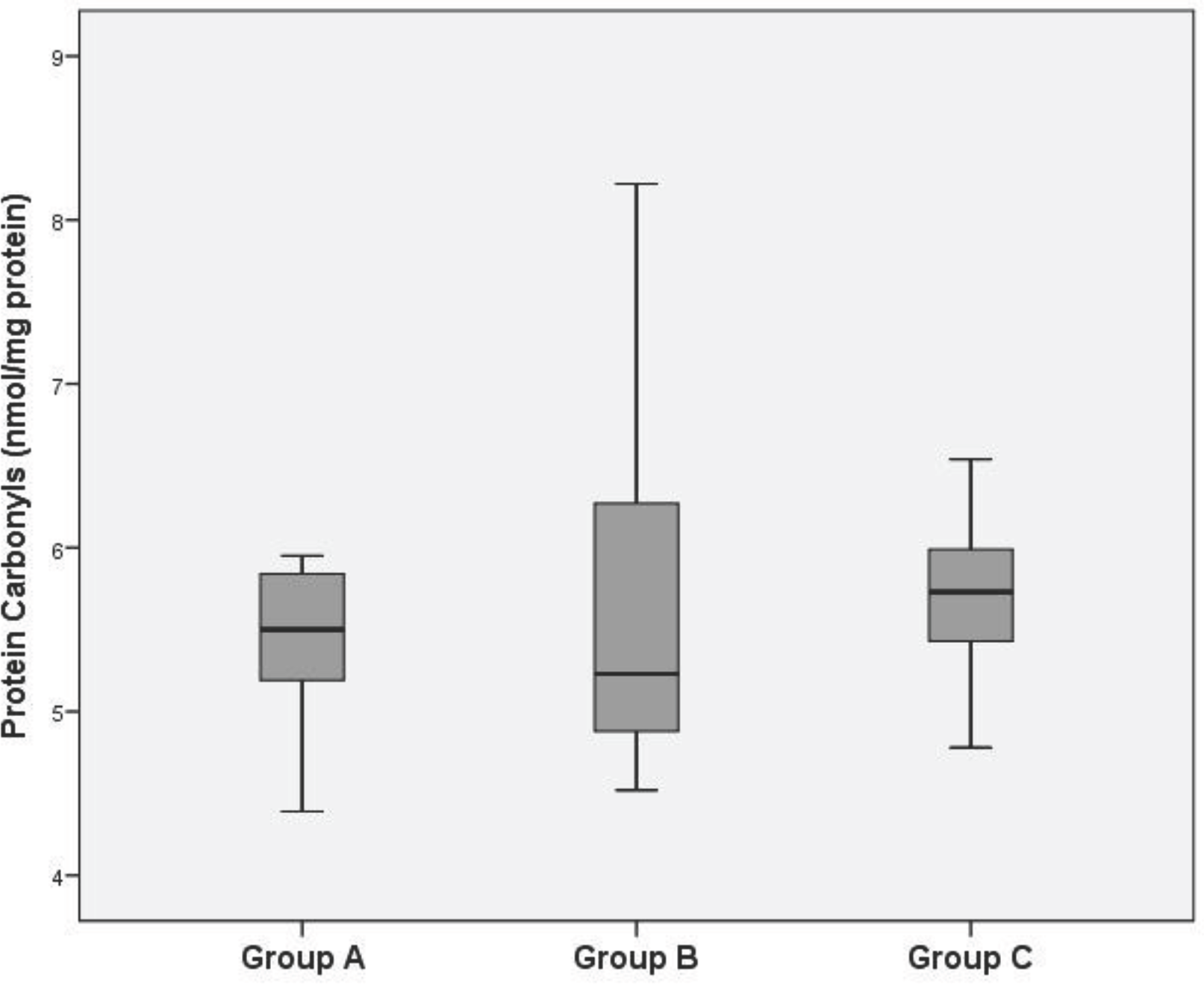
Lipid peroxide levels in the venous plasma of group B and C were significantly higher than those of group A (group B: 4.59±0.30, group C: 5.40±0.22 vs. group A: 3.90±0.26 nmol/mg protein, P<0.05). Lipid peroxide levels in the venous plasma of group C were significantly higher than those of group B (P<0.05). Protein carbonyl levels in the venous plasma of group C were significantly higher than those of group A (group C: 5.68±0.42 vs. group A: 5.43±0.41 nmol/mg protein, P<0.05). There was no significant difference of protein carbonyl levels in the venous plasma between group A and B. ORAC levels in the venous plasma of group B and C were significantly lower than those of group A (group B: 117.90±0.48, group C: 111.68±1.23 vs. group A: 119.14±1.12 mM/mL, P<0.05). ORAC levels in the venous plasma of group C were significantly lower than those of group B (P<0.05).
Conclusion
In the blood of the women with preterm premature rupture of membranes, the lipid peroxidation was increased and the antioxidant capacity was decreased compared to women with normal pregnancy and preterm labor with intact membranes. These results suggest that oxidative stress was increased in preterm premature rupture of membranes.
Go to : 
References
1. Mathews TJ, Menacker F, MacDorman MF, Centers for Disease Control and Prevention, National Center for Health Statistics. Infant mortality statistics from the 2002 period: linked birth/infant death data set. Natl Vital Stat Rep. 2004; 53:1–29.
2. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000; 342:1500–7.

3. Vercesi AE, Kowaltowski AJ, Oliveira HC, Castilho RF. Mitochondrial Ca2+ transport, permeability transition and oxidative stress in cell death: implication sincardiotoxicity, neurodegeneration and dyslipidemias. Front Biosci. 2006; 11:2554–64.
4. Bandy B, Davison AJ. Mitochondrial mutations may increase oxidative stress: implications for carcinogenesis and aging? Free Radic Biol Med. 1990; 8:523–39.

5. Connors N, Merrill D. Antioxidants for prevention of preterm delivery. Clin Obstet Gynecol. 2004; 47:822–32.

6. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012; 10:49.

7. Dennery PA. Oxidative stress in development: nature or nurture? Free Radic Biol Med. 2010; 49:1147–51.

8. Myatt L. Review: reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010; 31:66–9.

9. Torricelli M, Conti N, Galeazzi LR, Renzo GCD, Petraglia F, The Italian preterm network study group. Epidemiology of early preterm delivery: Relationship with clinical and histopathological infective parameters. J Obstet Gynaecol. 2013; 33:140–3.
10. Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014; 5:567.

11. Ohkawa H, Ohsihi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–8.

12. Oliver CN, Ahn B, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987; 262:5488–91.

13. Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993; 14:303–11.

14. Lockwood CJ, Kuczynski E. Markers of risk for preterm birth. J Perinat Med. 1999; 27:5–20.
15. Kacerovsky M, Musilova I, Khatibi A, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2012; 25:2014–19.

16. Cobo T, Kacerovsky M, Palacio M, et al. A prediction model of histological chorioamnionitis and funisitis in preterm prelabor rupture of membranes: analyses of multiple proteins in the amniotic fluid. J Matern Fetal Neonatal Med. 2012; 25:1995–2001.

17. Tsiartas P, Kacerovsky M, Musilova I, Homychova H, Cobo T, Savman K, et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2013; 26:1332–6.

18. Menon R, Polettini J, Syed TA, Saade GR, Boldogh I. Expression of 8-oxoguanine glycosylase in human fetal membranes. Am J Reprod Immunol. 2014; 72:75–84.

19. Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, et al. Senescence of primary amniotic cells via oxidative DNA damage. PLoS One. 2013; 8:e83416.

20. Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, et al. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 2012; 7:e31136.

21. Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2012; 29:273–83.

22. Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F. Prevalence and clinical significance of sterileintraamniotic inflammation in patients with preterm labor and intact membranes. AmJ Reprod Immunol. 2014; 72:458–74.
23. Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988; 12:262–79.
Go to : 
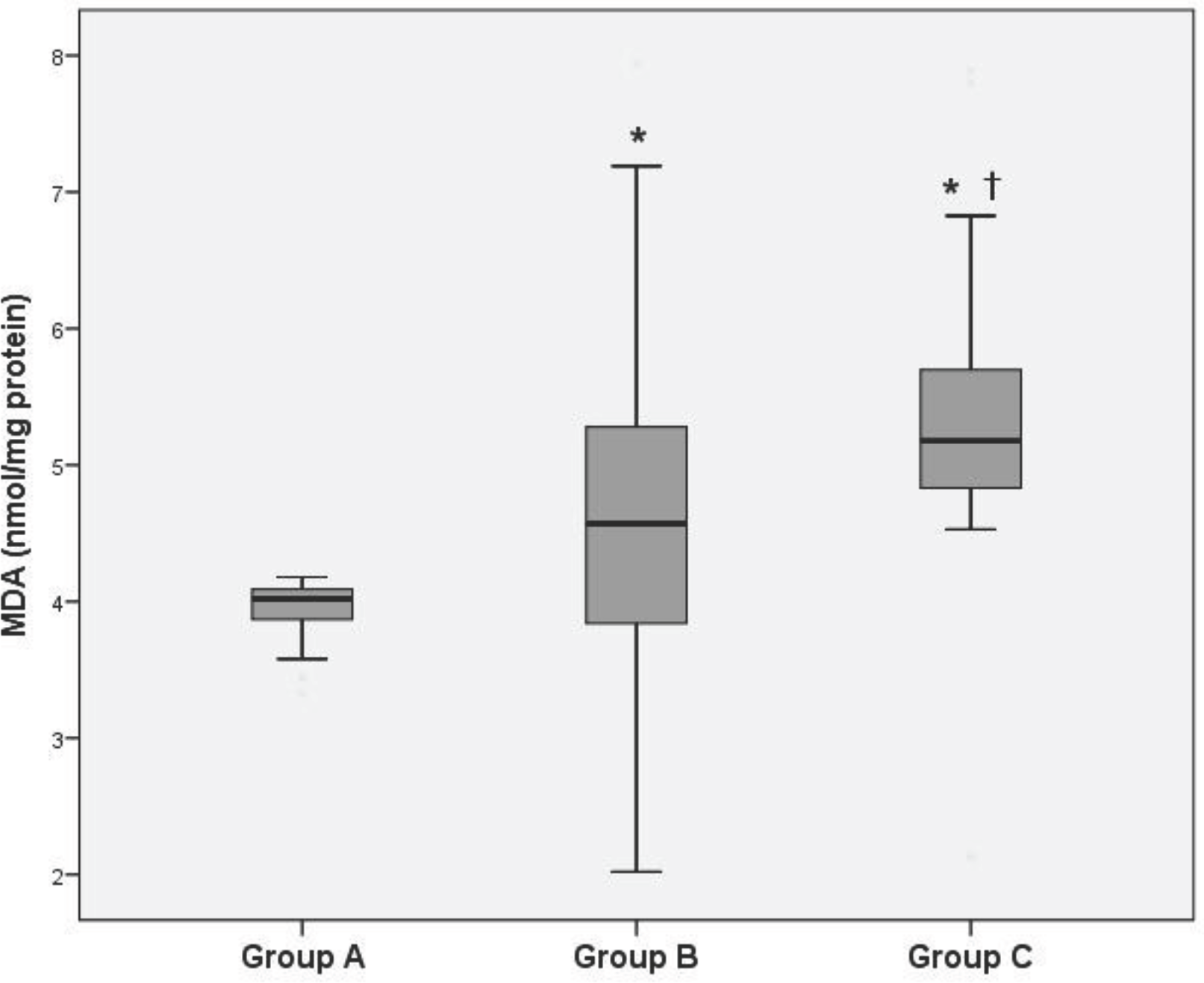 | Fig. 1.Levels of lipid peroxidation as estimated by thiobarbituric acid reaction in maternal venous plasma. ∗P<0.05 compared to Group A, †P<0.05 compared to Group B. Abbreviation: MDA, malondialdehyde. |
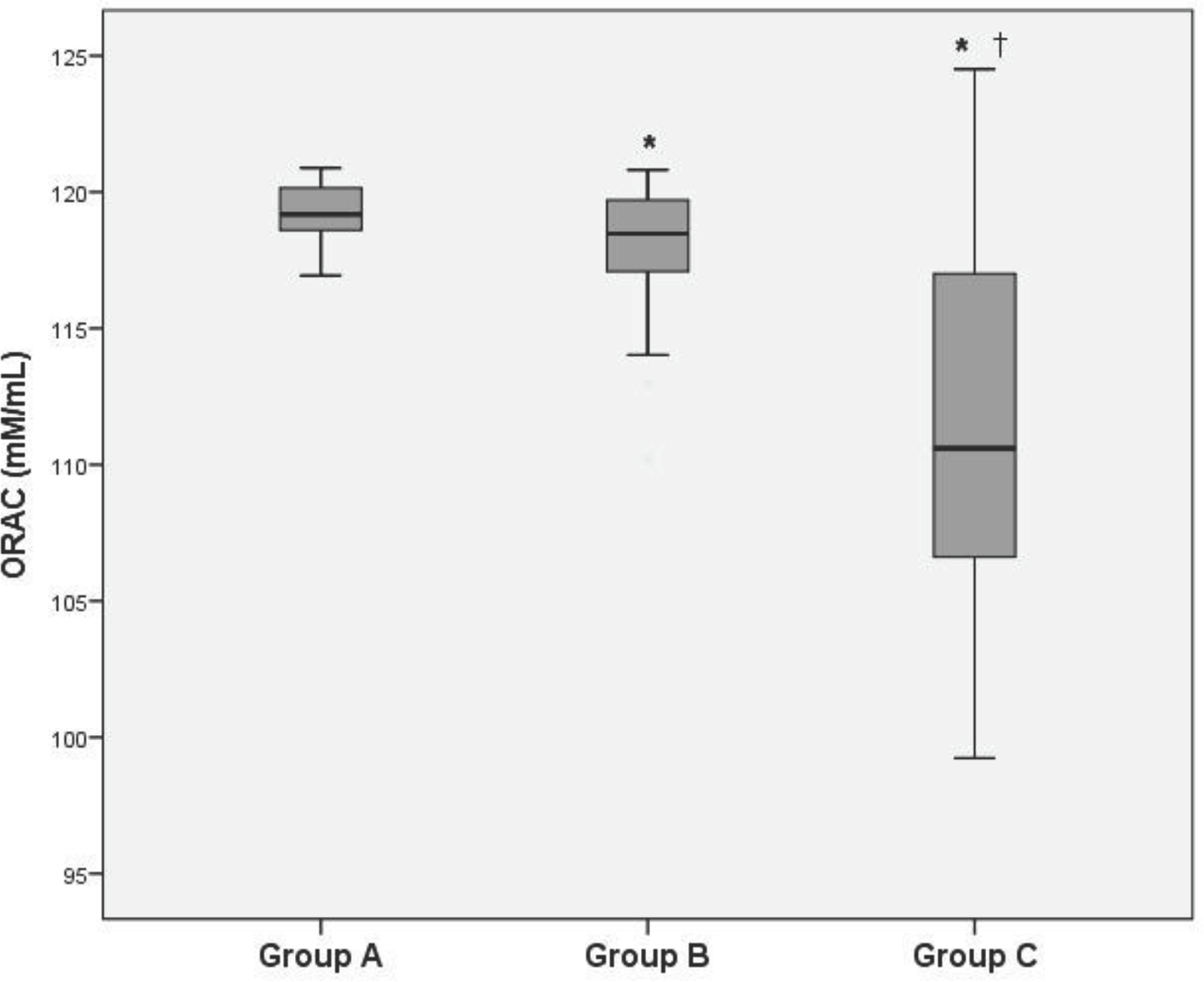 | Fig. 3.The oxygen radical absorbance capacity (ORAC) levels in maternal venous plasma. ∗P<0.05 compared to Group A, †P<0.05 compared to Group B. |
Table 1.
Clinical Characteristics and Oxidative Stress Markers of Two Groups
Table 2.
Clinical Characteristics and Oxidative Stress Markers of Patients with Latent Period below 3 Days




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download