Abstract
Survival of extreme preterm birth infants had recently been increasing steadily. Proper counseling and optimal management of women with impending periviable birth is one of the most intricate situations in both obstetricians and pediatricians. This article aimed 1) to discern several international recommendations on perinatal care of periviable birth proposed recently, 2) to provide reviews of best available evidence on the use of antenatal corticosteroids and magnesium sulfate in impending periviable birth, and 3) to present the results from survey on the obstetrical management in periviable birth targeting maternal-fetal medicine faculty members of the tertiary hospitals in our country.
Go to : 
References
1. Vohr BR. Neurodevelopmental outcomes of extremely preterm infants. Clin Perinatol. 2014; 41:241–55.

2. Haward MF, Kirshenbaum NW, Campbell DE. Care at the edge of viability: medical and ethical issues. Clin Perinatol. 2011; 38:471–92.

3. Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000; 343:378–84.
4. Bodeau-Livinec F, Marlow N, Ancel PY, Kurinczuk JJ, Costeloe K, Kaminski M. Impact of intensive care practices on short-term and longterm outcomes for extremely preterm infants: comparison between the British Isles and France. Pediatrics. 2008; 122:1014–21.

5. Doyle LW, Roberts G, Anderson PJ. Outcomes at age 2 years of infants <28 weeks'gestational age born in Victoria in 2005. J Pediatr. 2010; 156:49–53.
6. Field DJ, Dorling JS, Manktelow BN, Draper ES. Survival of extremely premature babies in a geographically defined population: prospective cohort study of 1994–9 compared with 2000–5. BMJ. 2008; 336:1221–3.

7. Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998–2003. Neonatology. 2010; 97:329–38.

8. Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity: moving beyond gestational age. N Engl J Med. 2008; 358:1672–81.
9. Fellman V, Hellström-Westas L, Norman M, Westgren M, Källén K, Lagercrantz H, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009; 301:2225–33.

10. Hintz SR, Poole WK, Wright LL, Fanaroff AA, Kendrick DE, Laptook AR, et al. Changes in mortality and morbidities among infants born at less than 25 weeks during the post-surfactant era. Arch Dis Child Fetal Neonatal Ed. 2005; 90:128–33.

11. Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M. Outcomes of infants born at 22 and 23 weeks'gestation. Pediatrics. 2013; 132:62–71.
12. Itabashi K, Horiuchi T, Kusuda S, Kabe K, Itani Y, Nakamura T. Mortality rates for extremely low birth weight infants born in Japan in 2005. Pediatrics. 2009; 123:445–50.

13. Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012; 345:e7976.

14. Manktelow BN, Seaton SE, Field DJ, Draper ES. Population-based estimates of in-unit survival for very preterm infants. Pediatrics. 2013; 131:e425–32.

15. ACOG practice bulletin. Perinatal care at the threshold of viability. Number 38, September 2002. American College of Obstetrics and Gynecology. Int J Gynaecol Obstet. 2002; 79:181–8.
16. Chauhan SP, Ananth CV. Periviable births: epidemiology and obstetrical antecedents. Semin Perinatol. 2013; 37:382–8.

17. Raju TN, Mercer BM, Burchfield DJ, Joseph GF Jr. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Am J Obstet Gynecol. 2014; 210:406–17.
18. Pignotti MS, Donzelli G. Perinatal care at the threshold of viability: an international comparison of practical guidelines for the treatment of extremely preterm births. Pediatrics. 2008; 121:e193–8.

19. Fetus and Newborn Committee, Canadian Paediatric Society, Maternal-Fetal Medicine Committee, Society of Obstetricians and Gynaecologists of Canada. Management of the woman with threatened birth of an infant of extremely low gestational age. CMAJ. 1994; 151:547–53.
20. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 38: perinatal care at the threshold of viability. Obstet Gynecol. 2002; 100:617–24.
21. NuffieldCouncilonBioethics.Criticalcaredecisionsinfetal andneonatalmedicine. Available at:. http://nuffieldbioethics.org/wp-content/uploads/2014/07/Conclusions-and-recommendations.pdf.
22. Tomlinson MW, Kaempf JW, Ferguson LA, Stewart VT. Caring for the pregnant woman presenting at periviable gestation: acknowledging the ambiguity and uncertainty. Am J Obstet Gynecol. 2010; 202:529.e1–6.

23. Raju TN, Mercer BM, Burchfield DJ, Joseph GF Jr. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014; 123:1083–96.
24. RajuTN, Mercer BM, Burchfield DJ, Joseph GF. Periviable birth: executive summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. J Perinatol. 2014; 34:333–42.
26. Gonzales LW, Ballard PL, Ertsey R, Williams MC. Glucocorticoids and thyroid hormones stimulate biochemical and morphological differentiation of human fetal lung in organ culture. J Clin Endocrinol Metab. 1986; 62:678–91.

27. Carlo W, McDonaldS , FanaroffA , Vohr BR, Stoll BJ, Ehrenkranz RA. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25weeks gestation. J Am Med Assoc. 2011; 306:2348–58.
28. Mori R, Kusuda S, Fujimura M. Neonatal Research Network Japan. Antenatal corticosteroids promote survival of extremely preterm infants born at 22 to 23 weeks of gestation. J Pediatr. 2011; 159:110–4.

29. Elimian A, Garry D, Figueroa R, Spitzer A, Wiencek V, Quirk JG. Antenatal betamethasone compared with dexamethasone (betacode trial): a randomized controlled trial. Obstet Gynecol. 2007; 110:26–30.

30. Lee BH, Stoll BJ, McDonald SA, Higgins RD. Adverse neonatal outcomes associated with antenatal dexamethasone versus antenatal betamethasone. Pediatrics. 2006; 117:1503–10.

31. Lee BH, Stoll BJ, McDonald SA, Higgins RD. Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone. Pediatrics. 2008; 121:289–96.

32. Sibai BM. Magnesium sulfate for neuroprotection in patients at risk for early preterm delivery: not yet. Am J Obstet Gynecol. 2011; 205:296–7.

33. Pryde PG, Mittendorf R. Contemporary usage of obstetric magnesium sulfate: indication, contraindication, and relevance of dose. Obstet Gynecol. 2009; 114:669–73.
34. Costantine MM, Weiner SJ. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a metaanalysis. Obstet Gynecol. 2009; 114:354–64.
35. American College of Obstetricians and Gynecologists Committee on Obstetric Practice; Society for Maternal-Fetal Medicine. Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010; 115:669–71.
36. Patient safety checklist no. 7: magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2012; 120:432–3.
37. The Antenatal Magnesium Sulphate for Neuroprotection Guideline Development Panel. Antenatal magnesium sulphate prior to preterm birth for neuroprotection of the fetus, infant and child: National clinical practice guidelines (2010). Available at:. http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp128_mag_sulphate_child.pdf.
38. Rattray BN, Kraus DM, Drinker LR, Goldberg RN, Tanaka DT, Cotten CM. Antenatal magnesium sulfate and spontaneous intestinal perforation in infants less than 25 weeks gestation. J Perinatol. 2014; 34:819–22.

39. Borja-Del-Rosario P, Basu SK, Haberman S, Bhutada A, Rastogi S. Neonatal serum magnesium concentrations are determined by total maternal dose of magnesium sulfate administered for neuroprotection. J Perinat Med. 2014; 42:207–11.

40. Basu SK, Chickajajur V, Lopez V, Bhutada A, Pagala M, Rastogi S. Immediate clinical outcomes in preterm neonates receiving antenatal magnesium for neuroprotection. J Perinat Med. 2011; 40:185–9.

41. Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003; 290:2669–76.

Go to : 
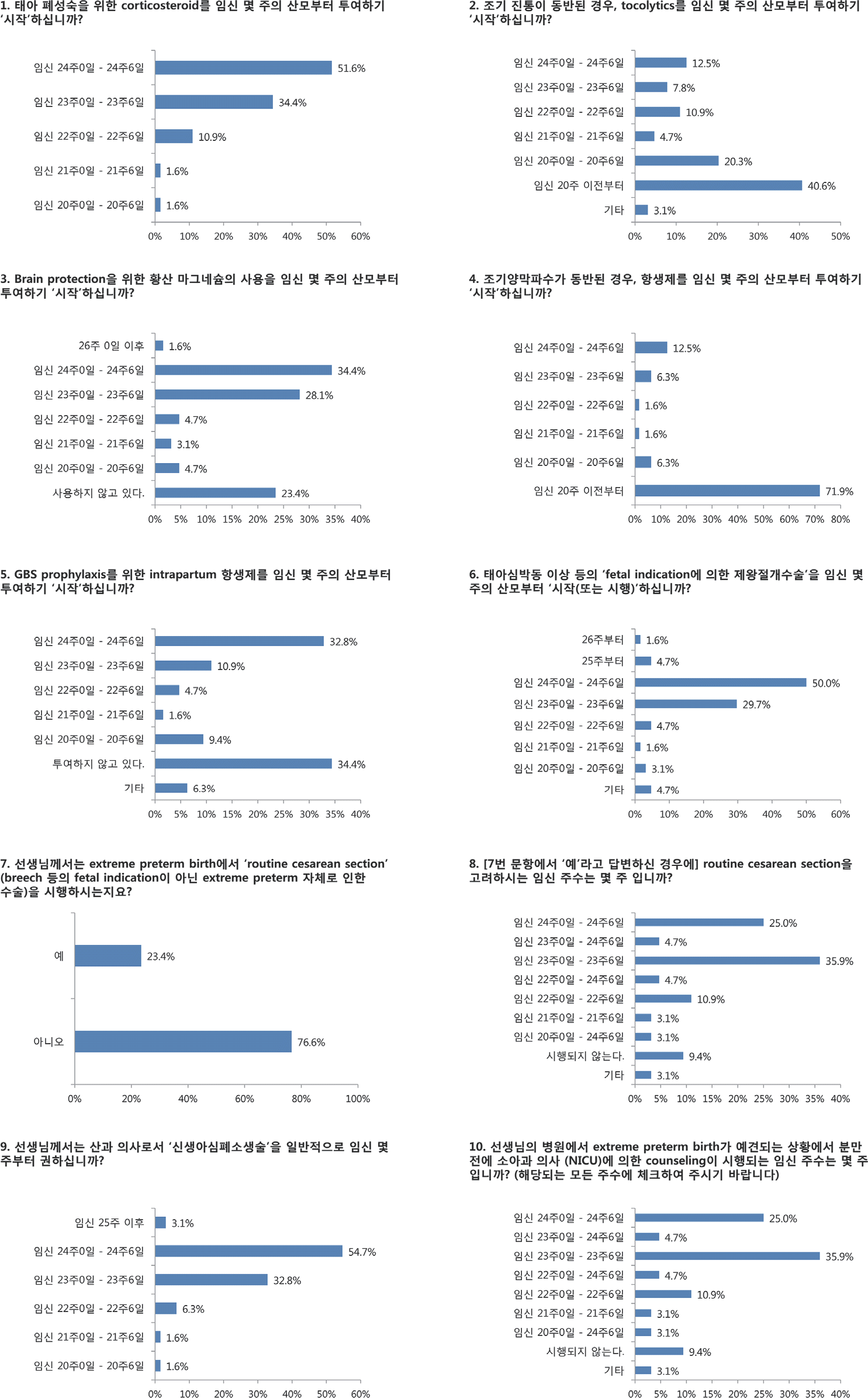 | Fig. 1.The questionnaires and responses from the survey regarding obstetric care on periviable birth. |
Table 1.
Survival rates in extreme preterm birth1
Table 2.
International comparison of practical guidelines for the treatment of extreme preterm births
Abbreviations: ACS, antenatal corticosteroids; -, data not available; C/S, cesarean section; Ix, indication; CPR, cardiopulmonary resuscitation; C/W, consistent with; w/o, without; Mx, management; ACOG, American College of Obstetricians and Gynecologists; NCB, Nuffield Counsil on Bioethics; UK, United Kingdom.
Table 3.
General guidance regarding obstetric interventions for threatened and imminent periviable birth17,23,24
Table 4.
Death or neurodevelopmental impairment by 18 to 22 months' corrected age for infants born at 22 to 25 weeks' gestation by exposure to antenatal corticosteroids27
| Steroid | No steroid | Adjusted OR∗ (95% CI) | |
|---|---|---|---|
| 22 weeks | 90.2 % (101/112) | 93.1 % (243/261) | 0.80 [0.29–2.21] |
| 23 weeks | 83.4 % (838/1005) | 90.5 % (676/747) | 0.58 [0.42–0.80] |
| 24 weeks | 68.4 % (1711/2502) | 80.3 % (559/696) | 0.62 [0.49–0.78] |
| 25 weeks | 52.7 % (1510/2865) | 67.9 % (451/664) | 0.61 [0.50–0.74] |
| Total | 64.2 % (4160/6484) | 81.5 % (1929/2368) | 0.60 [0.53–0.69] |
Table 5.
Comparison of three randomized trials of neuroprotection for preterm neonates: inclusion criteria, gestational age at entry, treatment regimens, median dose used and primary outcomes




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download