Abstract
Purpose
In this study, the risk factors of failure of ibuprofen treatment in preterm infants with hemodynamically significant patent ductus arteriosus (hsPDA) were investigated.
Methods
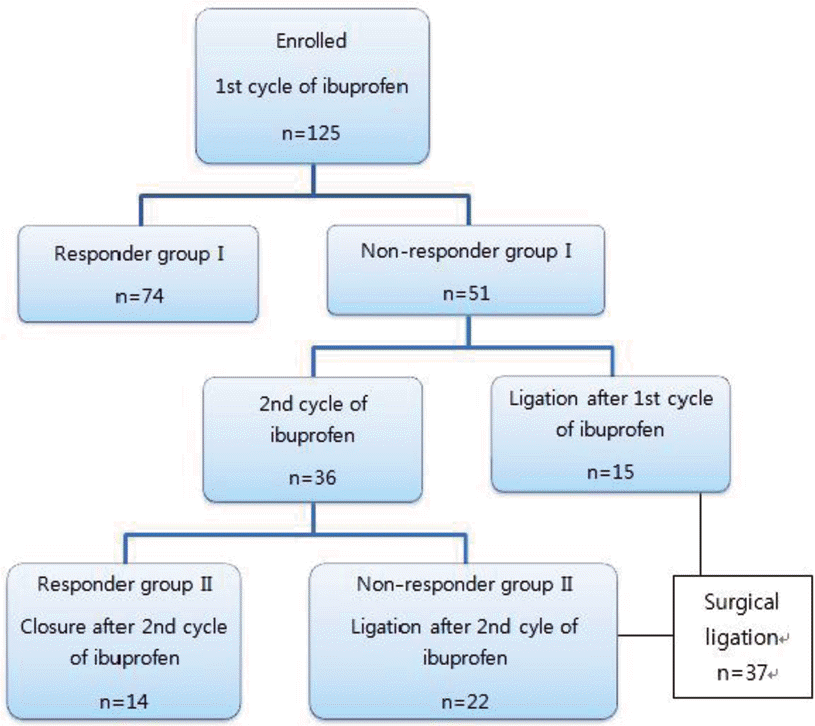
Among 403 preterm infants (<32 weeks gestation) born between January 2010 and December 2012, 125 infants treated with ibuprofen for hsPDA were retrospectively reviewed. The preterm infants were divided into the following groups according to their response to the 1st and 2nd cycles of ibuprofen treatment: responder groups I and II, closure of the ductus arteriosus after the 1st and 2nd cycles of ibuprofen treatment; and nonresponder groups I and II, persistency of hsPDA after the 1st and 2nd cycles of ibuprofen treatment.
Results
One hundred twenty five infants were enrolled in the study: 74 in responder group I, 51 in non-responder group I, 14 in responder group II, and 22 in non-responder group II. In non-responder group I, the gestational age and birth weight were smaller, the postnatal steroid treatment was more frequent, and the duration of mechanical ventilation and the days spent in the hospital were prolonged. In non-responder group II, the gestational age and birth weight were smaller, the diameters of the ductus arteriosus were larger, and the inotropics use was more frequent.
REFERENCES
1.Hoffman JI., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002. 39:1890–900.

2.Hermes-DeSantis ER., Clyman RI. Patent ductus arteriosus: pathophysiology and management. J Perinatol. 2006. 26(Suppl 1):14–8.

3.Koch J., Hensley G., Roy L., Brown S., Ramaciotti C., Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1,000 grams or less. Pediatrics. 2006. 117:1113–21.

5.Van Overmeire B., Smets K., Lecoutere D., Van de Broek H., Weyler J., Degroote K, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000. 343:674–81.

6.Vida VL., Lago P., Salvatori S., Boccuzzo G., Padalino MA., Milanesi O, et al. Is there an optimal timing for surgical ligation of patent ductus arteriosus in preterm infants? Ann Thorac Surg. 2009. 87:1509–15. discussion 1515-6.

7.Lee HJ., Sim GH., Jung KE., Lee JA., Choi CW., Kim EK, et al. Delayed closure effect in preterm infants with patent ductus arteriosus. Korean J Pediatr. 2008. 51:1065–70.

8.Yeh TF., Raval D., Luken J., Thalji A., Lilien L., Pildes RS. Clinical evaluation of premature infants with patent ductus arteriosus: a scoring system with echocardiogram, acid-base, and blood gas correlations. Crit Care Med. 1981. 9:655–7.
9.Desfrere L., Zohar S., Morville P., Brunhes A., Chevret S., Pons G, et al. Dose-finding study of ibuprofen in patent ductus arteriosus using the continual reassessment method. J Clin Pharm Ther. 2005. 30:121–32.

10.Lubchenco LO., Hansman C., Dressler M., Boyd E. Intrauterine Growth as Estimated from Liveborn Birth-Weight Data at 24 to 42 Weeks of Gestation. Pediatrics. 1963. 32:793–800.

11.Bevilacqua G., Chernev T., Parmigiani S., Iarakova N., Gaioni L., Volante E, et al. Use of surfactant for prophylaxis versus rescue treatment of respiratory distress syndrome: experience from an Italian-Bulgarian trial. Acta Biomed Ateneo Parmense. 1997. 68(Suppl 1):47–54.
12.Papile LA., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978. 92:529–34.

13.Walsh MC., Kliegman RM., Fanaroff AA. Necrotizing enterocolitis: a practitioner's perspective. Pediatr Rev. 1988. 9:219–26.

15.Su PH., Chen JY., Su CM., Huang TC., Lee HS. Comparison of ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Pediatr Int. 2003. 45:665–70.

16.Narayanan M., Cooper B., Weiss H., Clyman RI. Prophylactic indomethacin: factors determining permanent ductus arteriosus closure. J Pediatr. 2000. 136:330–7.

17.Clyman RI., Chan CY., Mauray F., Chen YQ., Cox W., Seidner SR, et al. Permanent anatomic closure of the ductus arteriosus in newborn baboons: the roles of postnatal constriction, hypoxia, and gestation. Pediatr Res. 1999. 45:19–29.

18.Weiss H., Cooper B., Brook M., Schlueter M., Clyman R. Factors determining reopening of the ductus arteriosus after successful clinical closure with indomethacin. J Pediatr. 1995. 127:466–71.

19.Adrouche-Amrani L., Green RS., Gluck KM., Lin J. Failure of a repeat course of cyclooxygenase inhibitor to close a PDA is a risk factor for developing chronic lung disease in ELBW infants. BMC Pediatr. 2012. 12:10.

20.Clyman R., Cassady G., Kirklin JK., Collins M., Philips JB 3rd. The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: reexamining a randomized con- trolled trial. J Pediatr. 2009. 154:873–6.
21.Jeon GW., Park SE., Choi CW., Hwang JH., Chang YS., Park WS. The Effects of Early Enteral Feeding in Extremely Low Birth-Weight Infants. Korean J Pediatr. 2005. 48:711–5.
Table 1.
Demographics and Obstetric Factors of Responder Goup I and Non-responder Group I
| Responder group I (n=74) | Non-responder group I (n=51) | P value | P value* | |
|---|---|---|---|---|
| Gestational age (wk+day) | 28+0±2+1 | 26+1±1+6 | <0.001 | |
| Birth weight (g) | 1,095±358 | 853±219 | <0.001 | |
| Male, n (%) | 29(39.2) | 20(39.2) | 1 | 0.588 |
| Apgar score at 1 min | 4.2+0.1 | 3.9+0.1 | 0.106 | 0.503 |
| Apgar score at 5 min | 6.8±0.1 | 6.4±0.1 | 0.016 | 0.471 |
| Cesarean section, n (%) | 49 (66.2) | 39 (76.5) | 0.238 | 0.014 |
| Multiple birth, n (%) | 18 (24.3) | 15 (29.4) | 0.542 | 0.982 |
| Antenatal steroid, n (%) | 56 (75.7) | 36 (70.6) | 0.542 | 0.899 |
| Maternal PIH, n (%) | 12 (16.2) | 8 (15.7) | 1 | 0.944 |
| Maternal DM, n (%) | 2 (2.7) | 0 (0) | 0.513 | 0.453 |
| Histologic chorioamnionitis, n (%) | 16 (21.6) | 9 (17.6) | 0.654 | 0.692 |
| Small for gestational age, n (%) | 8 (10.8) | 4 (7.8) | 0.76 | 0.293 |
| Initial ductal size (mm) | 2.59±0.77 | 2.81±0.57 | 0.027 | 0.159 |
| Age at ibuprofen administration (days) | 3.7±2.8 | 4.0±2.0 | 0.115 | 0.632 |
Table 2.
Demographics and Obstetric Factors of Responder group II and Non-responder Group II
| Responder group II (n=14) | Non-responder group II (n=22) | P value | P value* | |
|---|---|---|---|---|
| Gestational age (wk+day) | 27+4±1+5 | 25+3±1+4 | 0.001 | |
| Birth weight (g) | 1,031±221 | 768±189 | <0.001 | |
| Male, n (%) | 5 (35.7) | 7(31.8) | 1.0 | 0.922 |
| Apgar score at 1 min | 3.9+1.3 | 4.0+1.0 | 0.367 | 0.129 |
| Apgar score at 5 min | 6.5±1.1 | 6.3±1.0 | 0.181 | 0.010 |
| Cesarean section, n (%) | 11 (78.6) | 16 (72.7) | 1.0 | 0.911 |
| Multiple birth, n (%) | 4 (28.5) | 8 (36.4)) | 1.0 | 0.471 |
| Antenatal steroid, n (%) | 11 (78.6) | 13 (59.1) | 0.292 | 0.625 |
| Maternal PIH, n (%) | 2 (14.3) | 3 (13.6) | 1.0 | 0.893 |
| Maternal DM, n (%) | 0 (0) | 0 (0) | ||
| Histologic chorioamnionitis, n (%) | 1 (7.1) | 3 (22.7) | 0.370 | 0.122 |
| Small for gestational age, n (%) | 1 (7.1) | 2 (9.1) | 1.0 | 0.708 |
| Initial ductal size (mm) | 2.51±0.64 | 2.96±0.43 | <0.001 | 0.026 |
| Age at ibuprofen administration (days) | 4.5±2.2 | 3.8±1.8 | 0.179 | 0.333 |
Table 3.
Factors Associated with Patent Ductus Arteriosus of Responder Group I and Non-responder Group I
| Responder group I (n=74) | Non-responder group I (n=51) | P value | P value* | |
|---|---|---|---|---|
| RDS, n (%) | 74 (100) | 51 (100) | 1 | 1 |
| IVH (≥grade III), n (%) | 10 (13.5) | 11 (21.6 | 0.33 | 0.756 |
| Sepsis, n (%) | 13 (17.6) | 13 (25.5) | 0.37 | 0.851 |
| NEC (≥grade II), n (%) | 3 (4.1) | 1 (2) | 0.645 | 0.161 |
| Duration of parenteral nutrition (days) | 17.7±1.5 | 26.2±1.5 | <0.001 | 0.266 |
| ROP, n (%) | 4 (5.4) | 10 (19.6) | 0.02 | 0.126 |
| Inotropics use, n (%) | 18 (24.3) | 23 (45.1) | 0.02 | 0.498 |
| BPD (≥moderate), n (%) | 5 (6.8) | 11 (21.6) | 0.027 | 0.123 |
| Duration of mechanical ventilation (days) | 16.5±18.1 | 36.8±20.3 | <0.001 | 0.002 |
| Postnatal steroid treatment, n (%) | 13 (17.6) | 31(60.8) | <0.001 | <0.001 |
| Hospital stay (days) | 65±24 | 91±23 | <0.001 | <0.001 |
| Death, n (%) | 5 (6.8) | 1(2.0) | 0.339 | 0.022 |
Table 4.
Factors Associated with Patent Ductus Arteriosus of Responder Group II and Non-responder Group II
| Responder group II (n=14) | Non-responder group I (n=22) | II P value | P value* | |
|---|---|---|---|---|
| RDS, n (%) | 14 (100) | 22 (100) | ||
| IVH (≥ gradeIII), n (%) | 2 (14.3) | 4 (18.2) | 1.0 | 0.282 |
| Sepsis, n (%) | 2 (14.3) | 4 (18.2) | 1.0 | 0.353 |
| NEC (≥ stageII), n (%) | 0 (0) | 0 (0) | ||
| Duration of parenteral nutrition (days) | 23.0±13.0 | 25.5±8.7 | 0.048 | 0.616 |
| ROP, n (%) | 1 (7.1) | 5 (22.7) | 0.370 | 0.353 |
| Inotropics use, n (%) | 1 (7.1) | 16 (72.7) | <0.001 | 0.033 |
| BPD (≥ moderate), n (%) | 1 (7.1) | 7 (31.8) | 0.115 | 0.315 |
| Duration of mechanical ventilaton (days) | 36.5±21.8 | 46.9±18.2 | <0.001 | 0.046 |
| Postnatal steroid treatment, n (%) | 6 (42.9) | 14 (63.6) | 0.307 | 0.955 |
| Hospital stay (days) | 91±26 | 98±25 | 0.007 | 0.561 |
| Death, n (%) | 0 (0) | 1 (4.5) | 1.0 | 0.714 |
Table 5.
Risk Factors for Non-responder Group I
Table 6.
Risk Factors for Non-responder Group II




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download