Abstract
Purpose :
This study was aimed to investigate the clinical efficacy of recombinant activated factor VII (rFVIIa) for patients with intractable postpartum hemorrhage.
Methods :
This was a retrospective study of ten patients who were treated with rFVIIa from July 2010 to February 2012 in one tertiary center. To evaluate each case, we used a standardized case record form. The primary outcome measures were response of rFVIIa, reduction of blood product requirement, changes of coagulation parameter. The response of rFVIIa was categorized to three groups: “complete responder”, “partial responder”, “poor responder”.
Results :
After the administration of rFVIIa, effect for bleeding was completely responded in 4 patients, partially responded in 6 patients, and poorly responded in none. A certain amount of reduction in blood product requirements was noted following rFVIIa administration, although no significant differences were observed statistically between before and after rFVIIa administration except RBC (P<0.01). Fibrinogen and INR were significantly reduced in all case types, but other coagulation parameters were not (P<0.01).
Go to : 
REFERENCES
1). Lusher JM. Recombinant factor VIIa (Novoseven) in the treatment of internal bleeding in patients with factor VIII and IX inhibitors. Haemostasis. 1996. 26(Suppl 1):124–30.
2). Lau P., Ong V., Tan WT., Koh PL., Hartman M. Use of Activated Recombinant Factor VII in Severe Bleeding - Evidence for Efficacy and Safety in Trauma, Postpartum Hemorrhage, Cardiac Surgery, and Gastrointestinal Bleeding. Transfus Med Hemother. 2012. 39:139–50.

3). Monroe DM., Hoffman M., Oliver JA., Roberts HR. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997. 99:542–7.

4). Hoffman M., Monroe DM., Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis. 1998. 9(Suppl 1):S61–5.
5). Mittal S., Watson HG. A critical appraisal of the use of recombinant factor VIIa in acquired bleeding conditions. Br J Haematol. 2006. 133:355–63.

6). Kenet G., Walden R., Eldad A., Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999. 354:1879.

7). Mayer SA., Brun NC., Begtrup K., Broderick J., Davis S., Diringer MN, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005. 352:777–85.

8). Friederich PW., Henny CP., Messelink EJ., Geerdink MG., Keller T., Kurth KH, et al. Effect of recombinant activated factor VII on perioperative blood loss in patients undergoing retropubic prostatectomy: a doubleblind placebo-controlled randomised trial. Lancet. 2003. 361:201–5.

9). Lodge JP., Jonas S., Oussoultzoglou E., Malago M., Jayr C., Cherqui D, et al. Recombinant coagulation factor VIIa in major liver resection: a randomized, placebo-controlled, double-blind clinical trial. Anesthesiology. 2005. 102:269–75.
10). Singi SR., Fernandez E., Pandya ST., Badrinath HR. Recombinant factor VIIa: use in fatal post partum hemorrhageIndian experience case series and review of literature. Indian J Hematol Blood Transfus. 2009. 25:1–5.
11). Moscardo F., Perez F., de la Rubia J., Balerdi B., Lorenzo JI., Senent ML, et al. Successful treatment of severe intra-abdominal bleeding associated with disseminated intravascular coagulation using recombinant activated factor VII. Br J Hematol. 2001. 114:174–6.
12). Haynes J., Laffan M., Plaat F. Use of recombinant activated factor VII in massive obstetric hemorrhage. Int J Obstet Anesth. 2007. 16:40–9.
13). Alfirevic Z., Elbourne D., Pavord S., Bolte A., Van Geijn H., Mercier F, et al. Use of recombinant activated factor VII in primary postpartum hemorrhage: the Northern European registry 2000-2004. Obstet Gynecol. 2007. 110:1270–8.

14). Ahonen J., Jokela R. Recombinant factor VIIa for life threatening post-partum hemorrhage. Br J Anaesth. 2005. 94:592–5.
15). Hossain N., Shansi T., Haider S., Soomro N., Khan NH., Memon GU, et al. Use of recombinant activated factor VII for massive postpartum hemorrhage. Acta Obstet Gynecol Scand. 2007. 86:1200–6.

16). Ahonen J., Jokela R., Korttila K. An open non-randomized study of recombinant activated factor VII in major postpartum hemorrhage. Acta Anaesthesiol Scand. 2007. 51:929–36.
17). Barillari G., Frigo MG., Casarotto M., Farnia A., Masse B., Wetzl R, et al. Use of recombinant activated factor VII in severe postpartum hemorrhage: data from the Italian Registry: a multicentric observational retrospective study. Thromb Res. 2009. 124:e41–7.
18). Franchini M., Lippi G., Franchi M. The use of recombinant activated factor VII in obstetric and gynaecological haemorrhage. BJOG. 2007. 114:8–15.

19). McMorrow RC., Ryan SM., Blunnie WP., Bowen M., Carton EG., Gardiner J, et al. Use of recombinant factor VIIa in massive post-partum haemorrhage. Eur J Anaesthesiol. 2008. 25:293–8.

20). Phillips LE., McLintock C., Pollock W., Gatt S., Popham P., Jankelowitz G, et al. Recombinant activated factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg. 2009. 109:1908–15.

21). Tanchev S., Platikanov V., Karadimov D. Administration of recombinant factor VIIa for the management of massive bleeding due to uterine atonia in the post-placental period. Acta Obstet Gynecol Scand. 2005. 84:402–3.

22). Kobayashi T., Nakabayashi M., Yoshioka A., Maeda M., Ikenoue T. Recombinant activated factor VII (rFVIIa/NovoSeven) in the management of severe postpartum haemorrhage: initial report of a multicentre case series in Japan. Int J Hematol. 2012. 95:57–63.

23). Jan JY., Lin SY., Lin CH., Lee CN., Fan SZ., Han YY. Recombinant activated factor VII as a promising adjuvant therapy for postpartum hemorrhage in the practice of obstetric anesthesia: experience from a university hospital in Taiwan. J Obstet Gynaecol Res. 2011. 37:901–7.

24). Welsh A., McLintock C., Gatt S., Somerset D., Popham P., Ogle R. Guidelines for the use of recombinant activated factor VII in massive obstetric haemorrhage. Aust N Z J Obstet Gynaecol. 2008. 48:12–6.

Go to : 
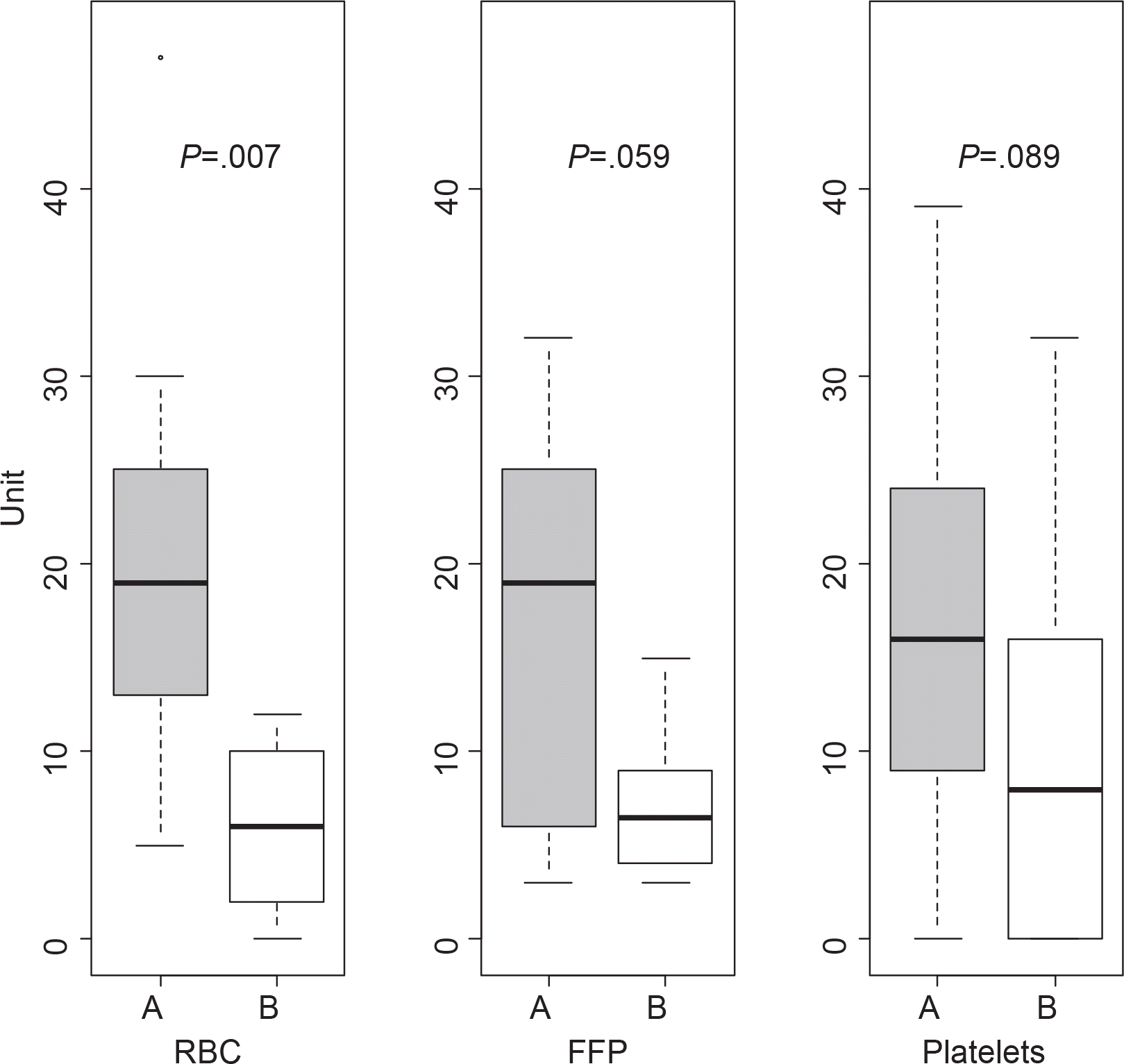 | Fig. 1Comparison of requirement of RBC, FFP and PLT concentrates before (A) and after (B) administration of rFVIIa. |
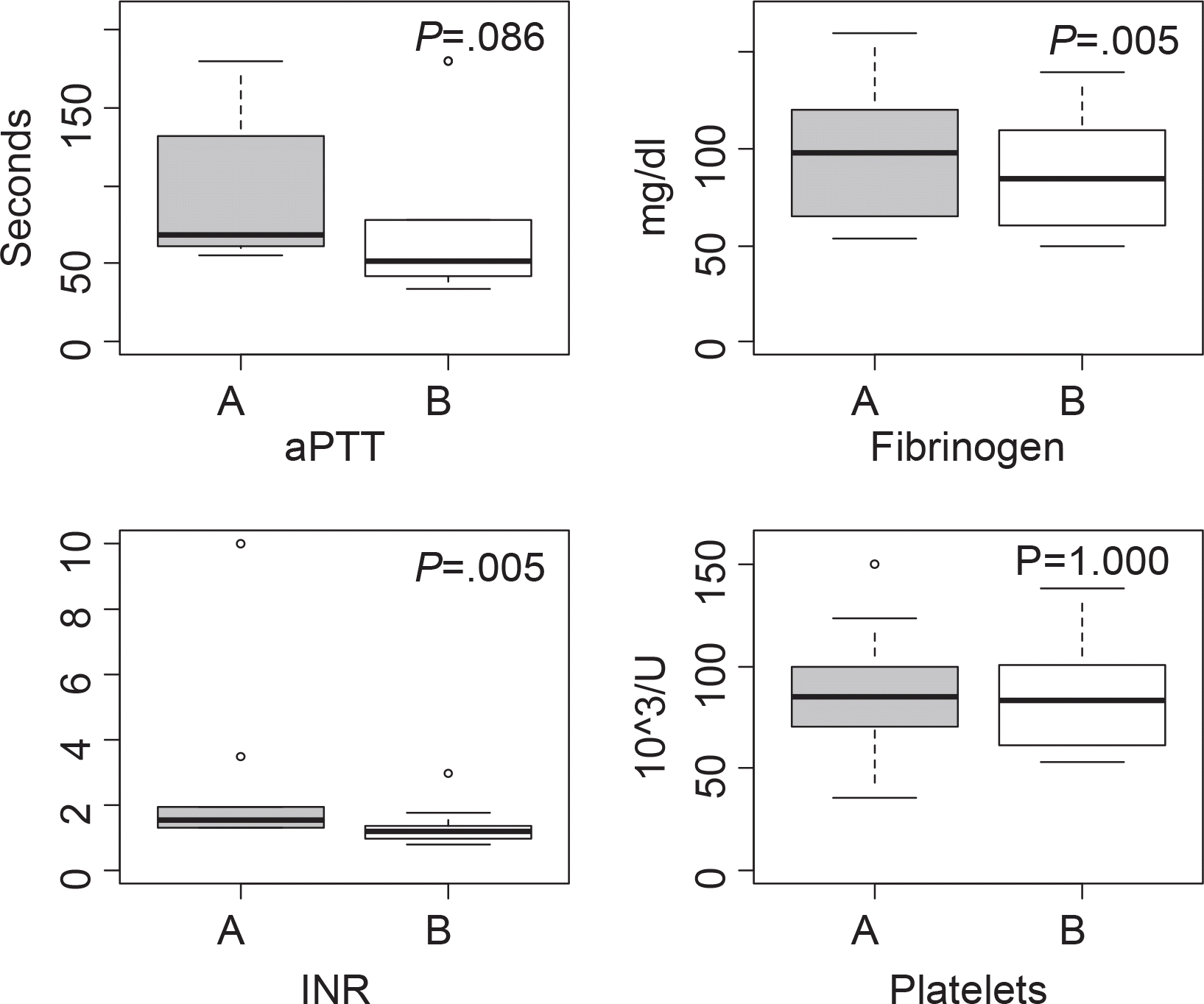 | Fig. 2Comparison of aPTT, fibrinogen, INR and platelets level before (A) and after (B) administration of rFVIIa. |
Table 1.
Patient Characteristics and Obstetric Data in Parturients Receiving rFVIIa
Table 2.
Comparison of Blood Products Requirement before and within 24 Hours after Initial rFVIIa Administration
Table 3.
Comparison of aPTT/INR, Fibrinogen and Platelet Level before and after Initial rFVIIa Administration




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download