Abstract
Purpose :
To evaluate the causes of nil per os (NPO) before reaching full enteral feeding and compare the clinical outcomes of extremely low birth weight infant (ELBWI) by NPO duration.
Methods :
We retrospectively reviewed the medical records of 92 ELBWI who were born and admitted to Neonatal intensive care unit (NICU) of Seoul National University Children's Hospital from January 2009 to December 2011. We analyzed the perinatal factors and causes of NPO. To compare neurodevelopmental outcomes and growth, we used K-ASQ (Korean ages & stages questionnaires) and growth Z-score.
Results :
There were total 163 fasting episodes before reaching full enteral feeding. Mean NPO time was 6.7± 5.6 days and mean frequency of NPO was 1.8 episodes. Most common cause of NPO was the medication for patent ductus arteriosus (PDA) closure (47.5%) and the next was the feeding intolerance (25.3%). Longer NPO group (more than 7 days) showed longer time to full enteral feeding and hospital day. Incidence of necrotizing enterocolitis was significantly higher in the longer NPO group. But there was no difference between two groups in the incidence of sepsis, cholestasis, and osteopenia. Changes in height Z-score from birth to postmenstrual age 35 weeks were significantly higher in the longer NPO group. In longer NPO group, catch-up of weight Z-score at CA 8 months was poor. And number of patients with score under cutoff level in K-ASQ was higher.
Go to : 
REFERENCES
1). Kliegman R., Nelson WE. Nelson textbook of pediatrics. 19th ed.Philadelphia PA: Elsevier/Saunders;2011. lxvii, 2610 p.p. 560–2.
2). Morgan J., Young L., McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane database Syst Rev. 2013. 5:CD001970.

3). Hernandez G., Velasco N., Wainstein C., Castillo L., Bugedo G., Maiz A, et al. Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care. 1999. 14:73–7.

4). Zingg W., Tomaske M., Martin M. Risk of parenteral nutrition in neonates—an overview. Nutrients. 2012. 4:1490–503.
5). Ben XM. Nutritional management of newborn infants: practical guidelines. World J Gastroenterol. 2008. 14:6133–9.

7). Walsh MC., Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986. 33:179–201.

8). Volpe JJ. Intraventricular Hemorrhage in the Premature-Infant - Current Concepts.1. Ann Neurol. 1989. 25:3–11.
10). Stephens BE., Walden RV., Gargus RA., Tucker R., McKinley L., Mance M, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009. 123:1337–43.

11). Fenton TR., Sauve RS. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr. 2007. 61:1380–5.

12). Kansagra K., Stoll B., Rognerud C., Niinikoski H., Ou CN., Harvey R, et al. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastro-intest Liver Physiol. 2003. 285:G1162–70.

13). Hadfield RJ., Sinclair DG., Houldsworth PE., Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995. 152:1545–8.

14). Jhaveri N., Soll RF., Clyman RI. Feeding practices and patent ductus arteriosus ligation preferences-are they related? Am J Perinatol. 2010. 27:667–74.

15). Van Bel F., Van Zoeren D., Schipper J., Guit GL., Baan J. Effect of indomethacin on superior mesenteric artery blood flow velocity in preterm infants. J Pediatr. 1990. 116:965–70.
16). Christmann V., Liem KD., Semmekrot BA., van de Bor M. Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin. Acta Paediatrica. 2002. 91:440–6.

17). Clyman RI., Couto J., Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012. 36:123–9.

18). Watterberg KL., Gerdes JS., Cole CH., Aucott SW., Thilo EH., Mammel MC, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. 2004. 114:1649–57.

19). Clyman R., Wickremasinghe A., Jhaveri N., Hassinger DC., Attridge JT., Sanocka U, et al. Enteral Feeding during Indomethacin and Ibuprofen Treatment of a Patent Ductus Arteriosus. J Pediatr. 2013. 163:406–11.

20). Bellander M., Ley D., Polberger S., Hellstrom-Westas L. Tolerance to early human milk feeding is not compromised by indomethacin in preterm infants with persistent ductus arteriosus. Acta Paediatrica. 2003. 92:1074–8.

21). Patole SK., Kumaran V., Travadi JN., Brooks JM., Doherty DA. Does patent ductus arteriosus affect feed tolerance in preterm neonates? Arch Dis Child Fetal Neonatal Ed. 2007. 92:F53–5.

22). Fanaro S. Strategies to improve feeding tolerance in preterm infants. J Matern Fetal Neonatal Med. 2012. 25(Suppl 4):54–6.

23). Patole S. Strategies for prevention of feed intolerance in preterm neonates: a systematic review. J Matern Fetal Neonatal Med. 2005. 18:67–76.

24). Tyson JE., Kennedy KA. Minimal enteral nutrition for promoting feeding tolerance and preventing morbidity in parenterally fed infants. Cochrane Database Syst Rev. 2000. CD000504.
25). Ng E., Shah VS. Erythromycin for the prevention and treatment of feeding intolerance in preterm infants. Cochrane Database Syst Rev. 2008. CD001815.

26). Oh S., Kim E-K., Neu J. Technologies for the Evaluation of Enteral Feeding Readiness in Premature Infants. Gastroenterology and Nutrition: Neonatology Questions and Contro- versies Series. 2012. 339.

27). Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007. 85:614S–20S.
Go to : 
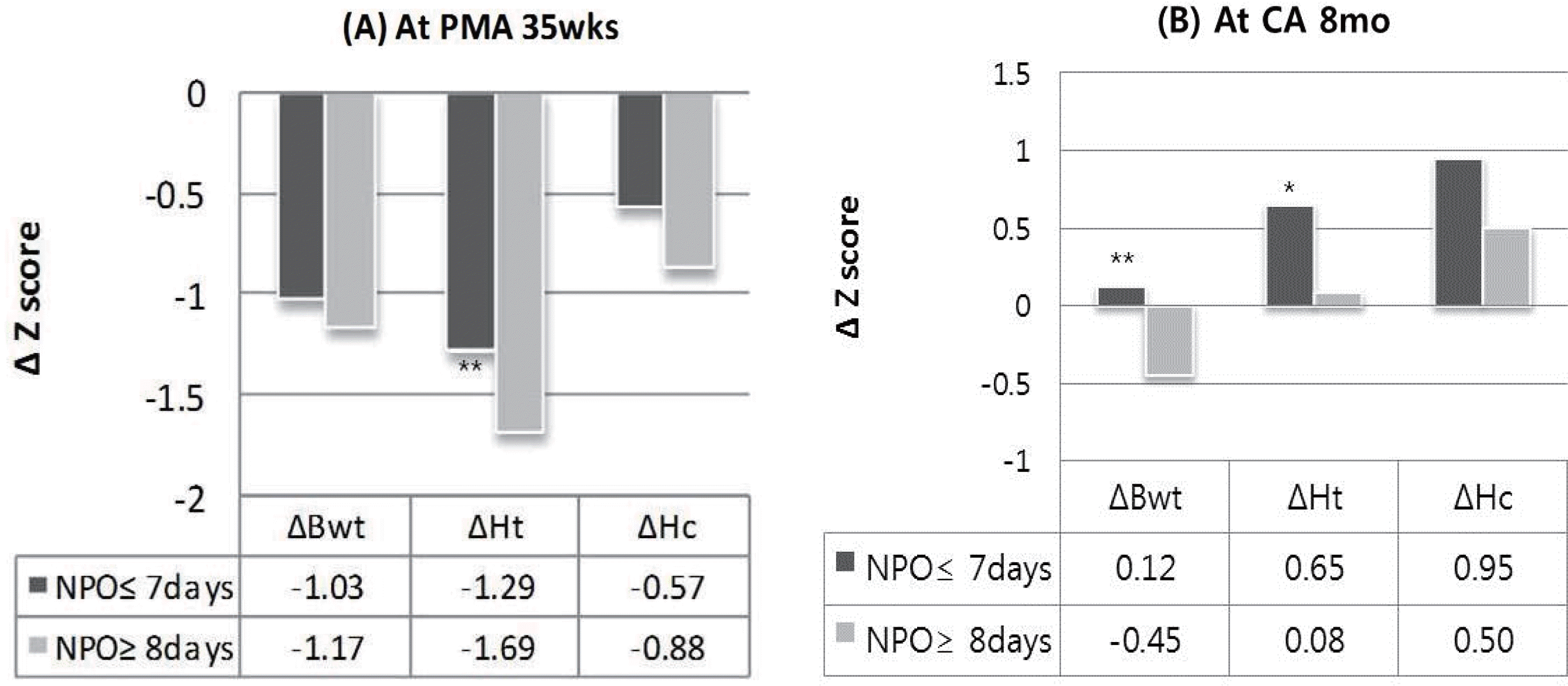 | Fig. 1(A) Growth Z score changes from birth at PMA 35weeks (∗∗P=0.049) (B) Growth Z score changes from birth at CA 8 month (∗P=0.07, ∗∗P=0.04) Abbreviations : Bwt; body weight, Ht; height, Hc; head circumference, PMA; postmenstrual age, CA; corrected age |
Table 1.
Causes of NPO before Full Enteral Feeding
Table 2.
Demographics and Clinical Characteristics of Study Population
Table 3.
Clinical Outcomes according to NPO Duration
| NPO ≤7 days (n=56) | NPO ≥8 days (n=36) | P value | |
|---|---|---|---|
| Duration of NPO, mean±SD, day | 3.6±2.0 | 11.6±6.0 | <0.01∗∗ |
| Frequency of NPO, mean±SD | 1.4±0.8 | 2.3±1.2 | <0.01∗∗ |
| Time to full enteral feeding, mean±SD, day | 17.2±5.3 | 26.9±8.9 | <0.01∗∗ |
| TPN duration, mean±SD, day | 19.6±11.6 | 26.4±8.8 | 0.040∗ |
| Hospital stay, mean±SD, day† | 71.8±24.0 | 86.3±24.0 | <0.01∗∗ |
| PMA at discharge, mean±SD, wk | 38.4±2.3 | 39.6±2.2 | 0.013∗ |
| Medication for PDA closure, n (%) | 43 (76.8) | 34 (94.4) | 0.025∗ |
| Culture proven sepsis, n (%) | 15 (26.8) | 11 (30.6) | 0.695 |
| NEC (≥stage 2), n (%) | 1 (1.8) | 5 (13.9) | 0.032∗ |
| BPD (≥moderate), n (%) | 15 (26.8) | 17 (47.2) | 0.107 |
| Cholestasis, n (%) | 14 (30.4) | 14 (40.0) | 0.370 |
| Osteopenia, n (%) | 23 (41) | 21 (58) | 0.106 |
| IVH (Gr≥3), n (%) | 0 (0) | 3 (8.3) | 0.057 |
| Cerebral palsy, n (%)‡ | 1 (1.9) | 4 (11.4) | 0.079 |
Table 4.
Calorie and Protein Intake during the First Week




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download