Abstract
Infectious complications such as necrotizing enterocolitis (NEC) or neonatal sepsis are main causes of mortality and disability in very low birth weight infants (VLBWIs). Because preterm infants are exposed to too unfavorable environment to promote appropriate intestinal colonization, pathologic organisms can easily colonize and host defense systems are also down-regulated in preterm gut, causing infectious complications such as NEC. To promote appropriate intestinal microbiota, probiotics have been studied for potential benefits by increasing mucosal barrier function, reducing intestinal pathogens, up-regulating immune system balancing inflammation. Large randomized controlled trials have shown favorable effects of probiotics on preterm NEC and mortality. Meta-analysis of clinical trials showed consistent evidence of probiotics uses in preterm infants. However, adequate type, dose, timing, and duration of probiotics have not been standardized for clinical applications. In addition, other interventions promoting preterm intestinal micobiota and immunity, such as prebiotics or lactoferrin, are on studies in combination with probiotics. More clinical trials should be added for the evidence-based clinical use of probiotics in preterm infants.
REFERENCES
1). Shah DK., Doyle LW., Anderson PJ., Bear M., Daley AJ., Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008. 153:170–5.

2). Hintz SR., Kendrick DE., Stoll BJ., Vohr BR., Fanaroff AA., Donovan EF, et al. NICHD Neonatal Research Network. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005. 115:696–703.

3). Patel RM., Denning PW. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: what is the current evidence? Clin Perinatol. 2013. 40:11–25.
4). OfekShlomai N., Deshpande G., Rao S., Patole S. Probiotics for Preterm Neonates: What Will It Take to Change Clinical Practice? Neonatology. 2013. 105:64–70.

5). Alfaleh K., Anabrees J., Bassler D. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: a meta-analysis. Neonatology. 2010. 97:93–9.

6). Mihatsch WA., Braegger CP., Decsi T., Kolacek S., Lanzinger H., Mayer B, et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin Nutr. 2012. 31:6–15.

7). Alfaleh K., Anabrees J., Bassler D., Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011. 16:CD005496.

8). MihatschWA. What is the power of evidence recommending routine probiotics for necrotizing enterocolitis prevention in preterm infants? Curr Opin Clin Nutr Metab Care. 2011. 14:302–6.
9). Wang Q., Dong J., Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg. 2012. 47:241–8.

10). Deshpande G., Rao S., Patole S., Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010. 125:921–30.

11). Stratiki Z., Costalos C., Sevastiadou S., Kastanidou O., Skour-oliakou M., Giakoumatou A, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev. 2007. 83:575–9.

12). Rougé C., Piloquet H., Butel MJ., Berger B., Rochat F., Ferraris L, et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2009. 89:1828–35.

13). Mihatsch WA., Vossbeck S., Eikmanns B., Hoegel J., Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology. 2010. 98:156–63.
14). Braga TD., da Silva GA., de Lira PI., deCarvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr. 2011. 93:81–6.

15). Sari FN., Dizdar EA., Oguz S., Erdeve O., Uras N., Dilmen U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr. 2011. 65:434–9.

16). Neu J., Shuster J. Non administration of routine probiotics unethical—really? Pediatrics. 2010. 126:e740–1.
17). Tarnow-Mordi WO., Wilkinson D., Trivedi A., Brok J. Probiotics reduce all-cause mortality and necrotizing enterocolitis: it is time to change practice. Pediatrics. 2010. 125:1068–70.

18). Deshpande G., Rao S., Patole S., Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010. 125:921–30.

20). Murguía-Peniche T., Mihatsch WA., Zegarra J., Supapannachart S., Ding ZY., Neu J. Intestinal mucosal defense system. Part 2. Probiotics and prebiotics. J Pediatr. 2013. 162:S64–71.

21). Berrington JE., Stewart CJ., Embleton ND., Cummings SP. Gut microbiota in preterm infants: assessment and relevance to health and disease. Arch Dis Child Fetal Neonatal Ed. 2013. 98:F286–90.

22). Bourlioux P., Koletzko B., Guarner F., Braesco V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium "The Intelligent Intestine," held in Paris, June 14, 2002. Am J Clin Nutr. 2003. 78:675–83.

23). Mshvildadze M., Neu J., Shuster J., Theriaque D., Li N., Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010. 156:20–5.

24). Neu J., Mihatsch WA., Zegarra J., Supapannachart S., Ding ZY., Murguía-Peniche T. Intestinal mucosal defense system, Part Consensus recommendations for immunonutrients. J Pediatr. 2013. 162:S56–63.
25). Preidis GA., Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009. 136:2015–31.

26). Claud EC., Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol. 2008. 42:S46–52.

27). Joint FAO/WHO Expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina. October 1-4. 2001.
28). Kitajima H., Sumida Y., Tanaka R., Yuki N., Takayama H., Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 1997. 76:F101–7.

29). Dani C., Biadaioli R., Bertini G., Martelli E., Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate. 2002. 82:103–8.
30). Costalos C., Skouteri V., Gounaris A., Sevastiadou S., Trianda-filidou A., Ekonomidou C, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev. 2003. 74:89–96.

31). Bin-Nun A., Bromiker R., Wilschanski M., Kaplan M., Rudensky B., Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005. 147:192–6.

32). Lin HC., Su BH., Chen AC., Lin TW., Tsai CH., Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005. 115:1–4.

33). Lin HC., Hsu CH., Chen HL., Chung MY., Hsu JF., Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008. 122:693–700.

34). Manzoni P., Mostert M., Leonessa ML., Priolo C., Farina D., Monetti C, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis. 2006. 42:1735–42.

35). Manzoni P., Rinaldi M., Cattani S., Pugni L., Romeo MG., Messner H, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, Italian Society of Neonatology. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009. 302:1421–8.
36). Samanta M., Sarkar M., Ghosh P., Ghosh Jk., Sinha Mk., Chatterjee S. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr. 2009. 55:128–31.

37). Fernández-Carrocera LA., Solis-Herrera A., Cabanillas-Ayón M., Gallardo-Sarmiento RB., García-Pérez CS., Montaño-Rodrí-guez R., Echániz-Aviles MO. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2013. 98:F5–9.
38). Jacobs SE., Tobin JM., Opie GF., Donath S., Tabrizi SN., Pirotta M, et al. ProPrems Study Group. Probiotic Effects on Late-onset Sepsis in Very Preterm Infants: A Randomized Controlled Trial. Pediatrics. 2013. 132:1055–62.
39). Sari FN., Eras Z., Dizdar EA., Erdeve O., Oguz SS., Uras N, et al. Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? Am J Perinatol. 2012. 29:579–86.

Fig. 2
Potentially harmful and potentially beneficial bacteria. P., Pseudomonas; E., Escherichia. Adopted from Bourlioux P, et al.
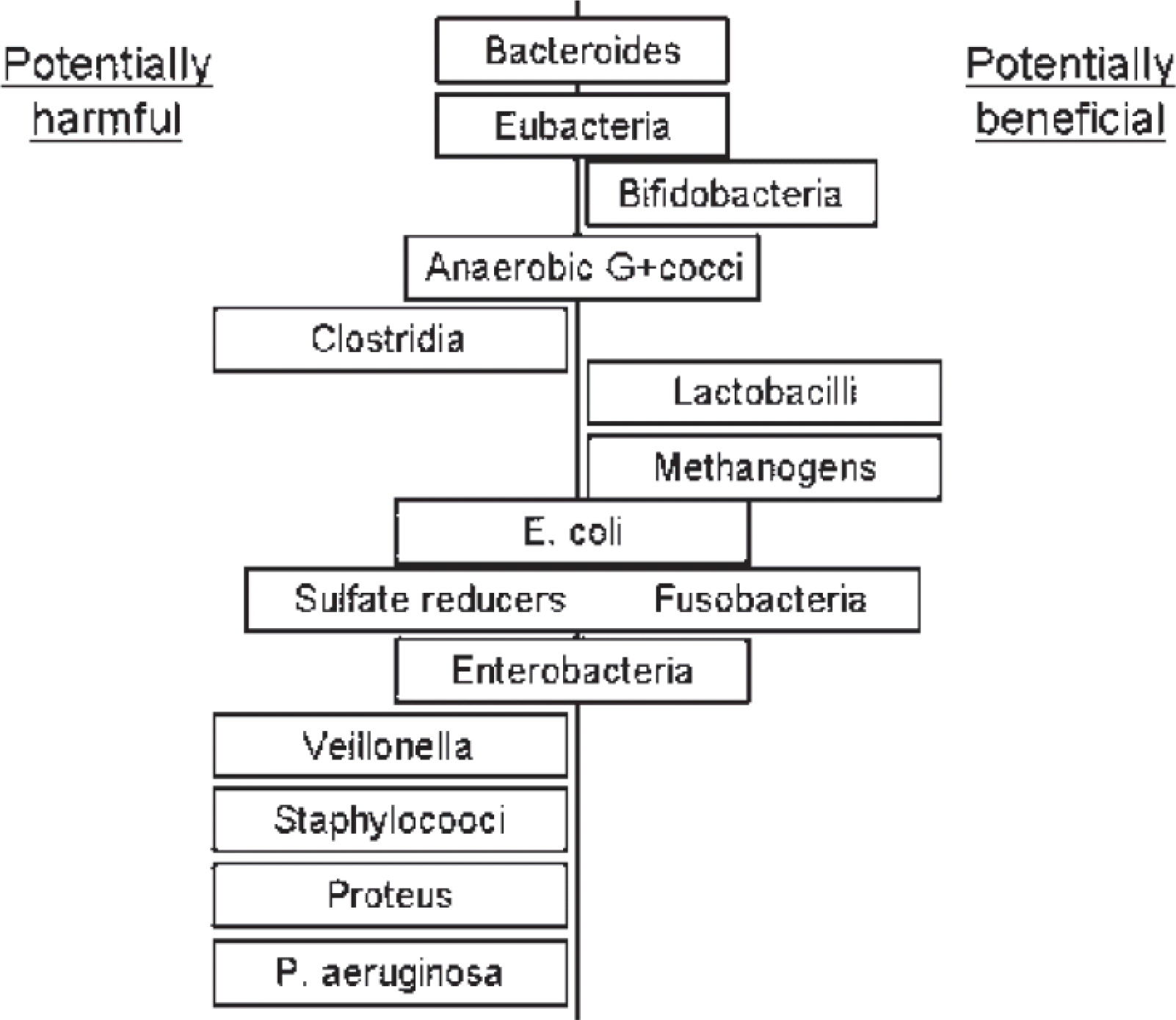
Table 1.
Probiotics Study of Very Low Birth Weight Infants for NEC, Sepsis, or Mortality Reduction
| Study | Case (n) | Control (n) | Bwt/GA | Probiotics | Dose(CFU)/duration |
|---|---|---|---|---|---|
| Kitajima28 | 45 | 46 | <1,500 g | B. breve | 0.5x109, 28 days |
| Dani29 | 295 | 290 | <33 wk or <1,500 g | L. GG | 6x109, till discharge |
| Costalos30 | 51 | 36 | 28-32 wk | S. boulardii | 109/kg, twice daily, 30 days |
| Bin-Nun31 | 72 | 73 | <1,500 g | Mixture∗ | till 36 wk |
| Lin32 | 180 | 187 | <1,500 g | Mixture† | twice daily, till discharge |
| Manzoni34 | 39 | 41 | <1,500 g | L. GG | 6x109, till discharge |
| Stratiki11 | 38 | 31 | <34 wk & <1,500 g | B. lactis | 2x107, 30 days |
| Lin33 | 217 | 217 | <34 wk & <1,500 g | Mixture‡ | 2x109, 6 wk |
| Manzoni35 | 151 | 168 | <1,500 g | L. GG | 6x109, till discharge |
| Rouge12 | 45 | 49 | <32 wk & <1,500 g | Mixture∮ | 1x108, till discharge |
| Samanta36 | 92 | 95 | <34 wk & <1,500 g | MixtureII | 2.5x109, till discharge |
| Mihatsch13 | 91 | 89 | <30 wk & <1,500 g | B. lactis | 12x109/kg, 6 wk |
| Braga14 | 119 | 112 | <1,500 g | Mixture¶ | 30 day of life |
| Sari15 | 110 | 111 | <33 wk or <1,500 g | L. sporogenes | 3.5x108, till discharge |
| Fernandez37 | 75 | 75 | <1,500 g | Mixture∗∗ | 1 g/day, till discharge |
| Jacobs38 | 548 | 551 | <32 wk & <1,500 g | Mixture†† | 109/1.5 g, till discharge |
Abbreviations: NEC, necrotizing enterocolitis; Bwt, birth weight; GA, gestational age; CFU, colny forming unit; B, Bifidobacterium; L,Lactobacillus; S,Saccharomyces ∗B. infantis (0.35x109)+Streptococcus thermophilus (0.35x109)+B. bifidus (0.35x109);




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download