Abstract
Purpose :
We aimed to describe the clinical features of late-onset circulatory collapse (LCC) in preterm infants.
Methods :
The records of preterm infants with a gestational age of <33 weeks who were admitted to a single neonatal intensive care unit and survived more than 72 hrs between March 2006 and August 2012 were reviewed retrospectively.
Results :
Of the total of 659 patients, 44 (6.7%) were diagnosed with LCC. Their mean gestational age was 26.0±1.9 weeks and their median birth weight 830 g. The median time of onset of LCC was 16.5 postnatal days. The patients exhibited oliguria that responded to hydrocortisone but not to hydration or catecholamines. Other clinical features of LCC were hypotension (73%), hyponatremia (52%), and hyperkalemia (34%). These abnormalities resolved in sequence: oliguria resolved first, after a median of 2.2 hrs, followed by hypotension after a median of 3.0 hrs, and the serum Na level became normal after 12.9 hrs. The incidence of LCC increased as the gestational age and/or birth weight decreased. A total of 26 patients (59%) developed LCC within 2 weeks after the initiation of levothyroxine therapy.
Go to : 
REFERENCES
1). Kuint J., Barak M., Morag I., Maayan-Metzger A. Early treated hypotension and outcome in very low birth weight infants. Neonatology. 2009. 95:311–6.

2). Martens SE., Rijken M., Stoelhorst GM., van Zwieten PH., Zwinderman AH., Wit JM, et al. Is hypotension a major risk factor for neurological morbidity at term age in very preterm infants? Early Hum Dev. 2003. 75:79–89.

3). Pladys P., Wodey E., Betremieux P., Beuchee A., Ecoffey C. Effects of volume expansion on cardiac output in the preterm infant. Acta Paediatr. 1997. 86:1241–5.

4). Gill AB., Weindling AM. Echocardiographic assessment of cardiac function in shocked very low birthweight infants. Arch Dis Child. 1993. 68:17–21.

5). Bauer K., Linderkamp O., Versmold HT. Systolic blood pressure and blood volume in preterm infants. Arch Dis Child. 1993. 69:521–2.

6). Ng PC., Lam CW., Fok TF., Lee CH., Ma KC., Chan IH, et al. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch Dis Child Fetal Neonatal Ed. 2001. 84:F122–4.

7). Fauser A., Pohlandt F., Bartmann P., Gortner L. Rapid increase of blood pressure in extremely low birth weight infants after a single dose of dexamethasone. Eur J Pediatr. 1993. 152:354–6.

8). Gaissmaier RE., Pohlandt F. Single-dose dexamethasone treatment of hypotension in preterm infants. J Pediatr. 1999. 134:701–5.

9). Heckmann M., Wudy SA., Haack D., Pohlandt F. Serum cortisol concentrations in ill preterm infants less than 30 weeks gestational age. Acta Paediatr. 2000. 89:1098–103.

10). Helbock HJ., Insoft RM., Conte FA. Glucocorticoid-responsive hypotension in extremely low birth weight newborns. Pediatrics. 1993. 92:715–7.

11). Watterberg KL., Scott SM. Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics. 1995. 95:120–5.

12). Thomas S., Murphy JF., Dyas J., Ryalls M., Hughes IA. Response to ACTH in the newborn. Arch Dis Child. 1986. 61:57–60.

13). Ng PC., Lee CH., Lam CW., Ma KC., Fok TF., Chan IH, et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2004. 89:119–26.

14). Kusuda S., Fujimura M., Sakuma I., Aotani H., Kabe K., Itani Y, et al. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics. 2006. 118:e1130–8.

15). Masumoto K., Kusuda S., Aoyagi H., Tamura Y., Obonai T., Yamasaki C, et al. Comparison of serum cortisol concentrations in preterm infants with or without late-onset circulatory collapse due to adrenal insufficiency of prematurity. Pediatr Res. 2008. 63:686–90.

16). Lee JA., Choi CW., Kim EK., Kim HS., Kim BI., Choi JH. Late-onset hypotension and late circulatory collapse due to adrenal insufficiency in preterm infants with gestational age less than 32 weeks. J Korean Soc Neonatol. 2011. 18:211–20.

17). Choi EJ., Sohn JA., Lee EH., Lee JY., Lee HJ., Chung HR, et al. Clinical picture of adrenal insufficiency-associated hypotension in preterm infants. J Korean Soc Neonatol. 2011. 18:82–8.

18). de Vries LS., Eken P., Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992. 49:1–6.

19). Volpe JJ. Neurology of the Newborn. 5th ed.Philadelphia: WB Saunders Co;2008. p. 517–88.
20). Bell MJ., Ternberg JL., Feigin RD., Keating JP., Marshall R., Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978. 187:1–7.
21). Nakanishi H., Yamanaka S., Koriyama T., Shishida N., Miyagi N., Kim TJ, et al. Clinical characterization and long-term prognosis of neurological development in preterm infants with late-onset circulatory collapse. J Perinatol. 2010. 30:751–6.

22). Kawai M., Kusuda S., Cho K., Horikawa R., Takizawa F., Ono M, et al. Nationwide surveillance of circulatory collapse associated with levothyroxine administration in very-low-birthweight SS infants in Japan. Pediatr Int. 2012. 54:177–81.
23). Munro MJ., Walker AM., Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004. 114:1591–6.

24). Akanishi H., Matsunami S., Koriyama T., Ehara E., Kim T., Kusada S. Late-onset circulatory collapse and late development of periventricular leukomalacia in infants less than 33 weeks gestational age. J Jpn Soc Prem Newborn Med. 2005. 17:57–67.
25). Takizawa F., Kashimada K., Enomoto K., Miyai K., Ono M., Asada G, et al. Two preterm infants with late onset circulatory collapse induced by levothyroxine sodium. Pediatr Int. 2010. 52:e154–7.

26). Kawai M., Kusuda S., Cho K., Horikawa R., Takizawa F., Ono M, et al. Nationwide surveillance of circulatory collapse associated with levothyroxine administration in very-low-birthweight infants in Japan. Pediat Int. 2012. 54:177–81.

27). Yagasaki H., Kobayashi K., Nemoto A., Naito A., Sugita K., Ohyama K. Late-onset circulatory dysfunction after thyroid hormone treatment in an extremely low birth weight infant. J Pediatr Endocrinol Metab. 2010. 23:153–8.

28). Watterberg KL., Shaffer ML., Mishefske MJ., Leach CL., Mammel MC., Couser RJ, et al. Growth and neurodevelop-mental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics. 2007. 120:40–8.

29). Kobayashi S., Fujimoto S., Koyama N., Fukuda S., Iwaki T., Tanaka T, et al. Late-onset circulatory dysfunction of premature infants and late-onset periventricular leukomalacia. Pediatr Int. 2008. 50:225–31.

30). Kobayashi S., Fujimoto S., Fukuda S., Hattori A., Iwaki T., Koyama N, et al. Periventricular leukomalacia with late-onset circulatory dysfunction of premature infants: correlation with severity of magnetic resonance imaging findings and neurological outcomes. The Tohoku Journal of Experimental Medicine. 2006. 210:333–9.

Go to : 
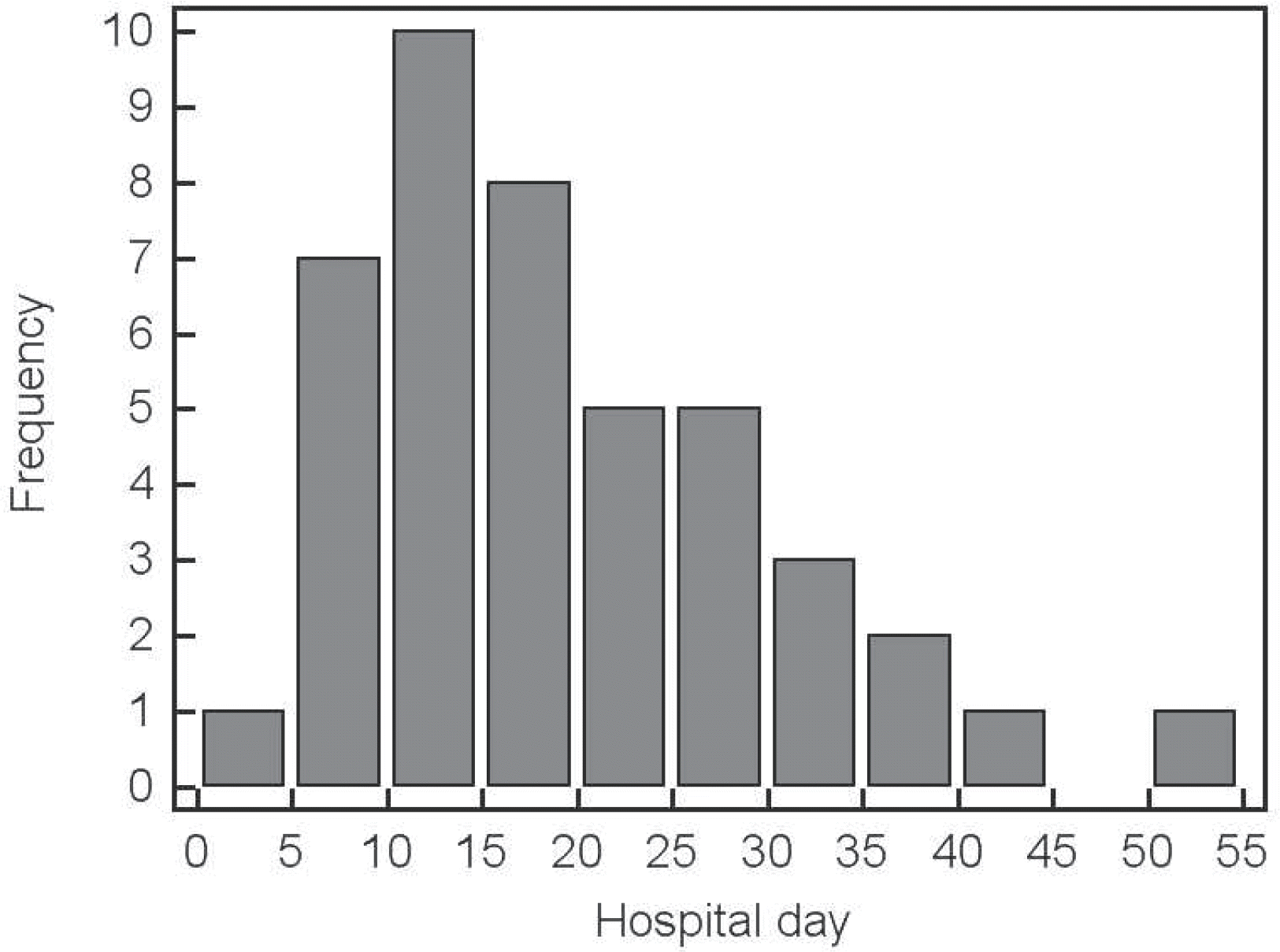 | Fig. 1Histogram of the onset of late-onset circulatory collapse showed that the onset peaked between the 11th and 15th days of life. The LCC developed between the 6th and 40th days of hospitalization in most cases. |
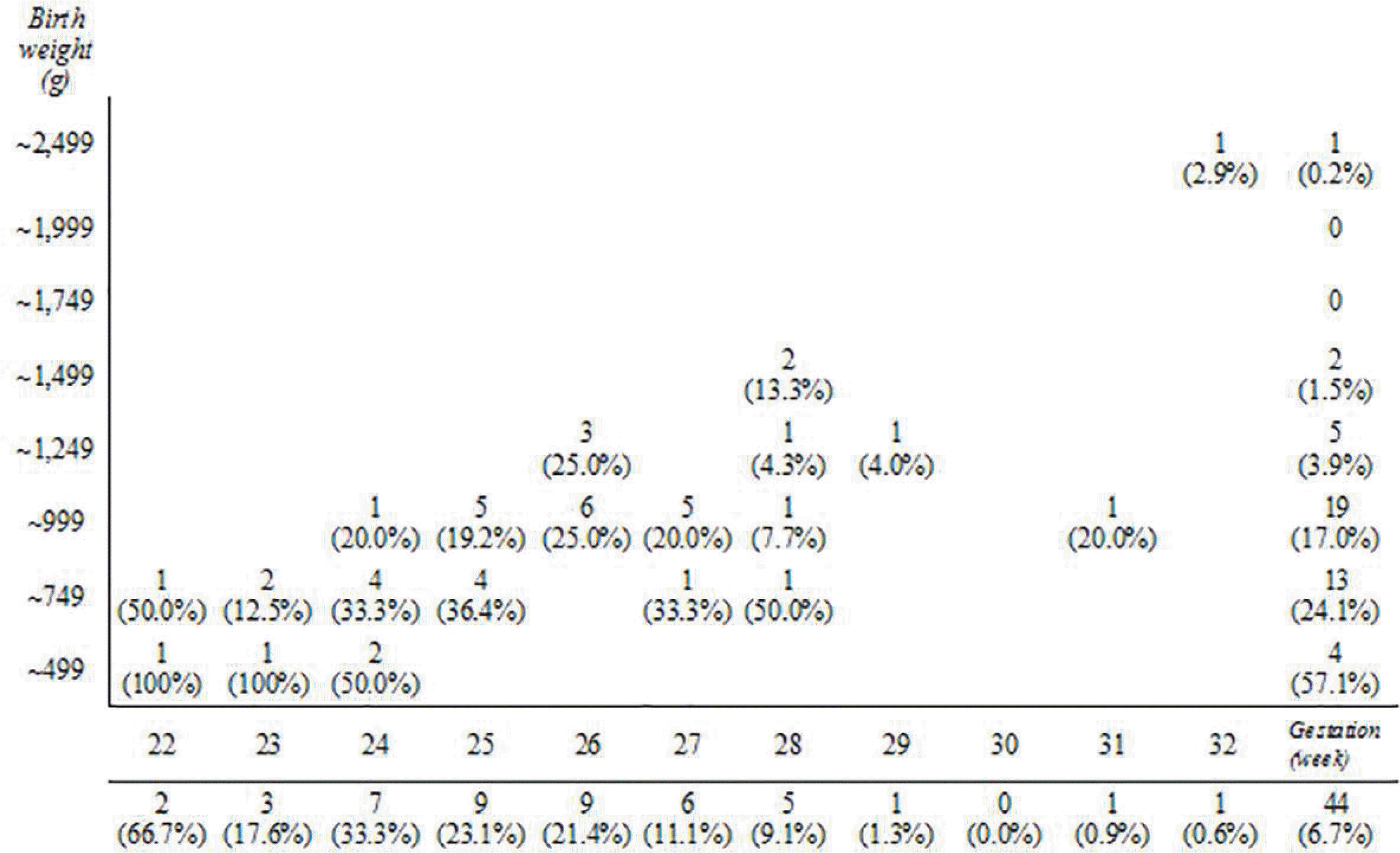 | Fig. 2The incidence of late-onset circulatory collapse increased as the gestational age decreased and the birth weight decreased. |
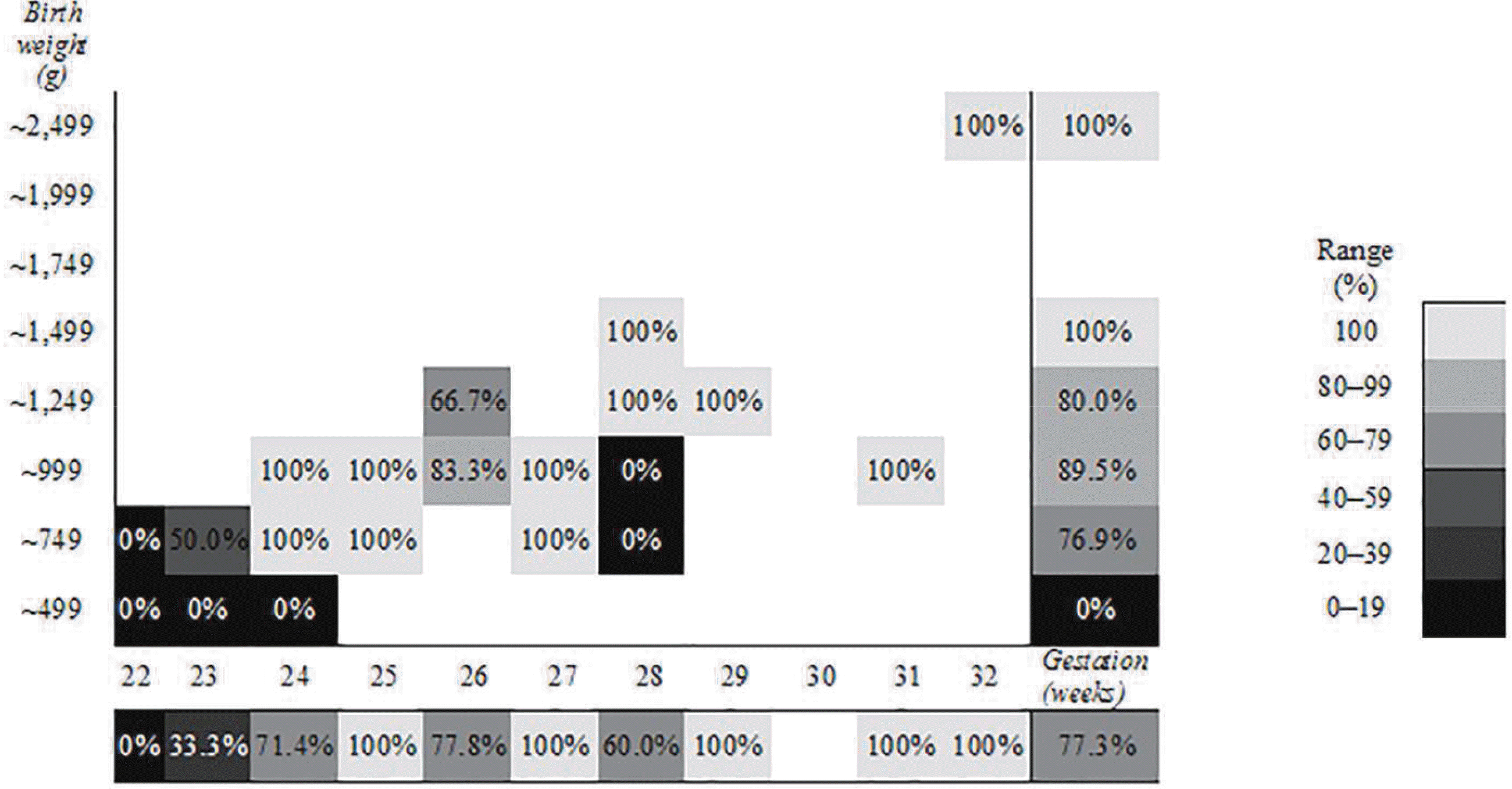 | Fig. 3There was no association between the survival rate of patients with late-onset circulatory collapse and the gestational age. The survival rate tended to decrease as the birth weight decreased. |
Table 1.
Major Vital Signs and Laboratory Findings of Infants with Late-Onset Circulatory Collapse




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download