Abstract
Purpose
CD74 (cluster of differentiation 74) is a type II transmembrane protein that associates with MHC-II (major histocompatibility complex-II) molecules and binds with the macrophage migration inhibitory factor (MIF), which is known to be associated with tumorigenesis. The purpose of this study was to examine the relationship between CD74 with MIF and the role of CD74 in osteosarcoma tumorigenesis.
Materials and Methods
A correlation between CD74 with MIF was examined using immunohistochemistry (IHC) of tissues from patients with osteosarcoma (n=45). The mRNA expression of CD74 from patient-derived primary cells of osteosarcoma (n=22) was examined by a real-time polymerase chain reaction. The role of CD74 and in cisplatin-resistance of osteosarcoma was examined using WST-1 and colony forming assay.
Results
Of the 45 patients, 25 patients (55.6%) had a high level of expression in both CD74 and MIF by IHC. High level of MIF expression was observed in 40 patients (88.9%). We detected that mRNA expression of CD74 was higher in cells of all osteosarcoma patients (n=22) examined than in the control cells (hFOb 1.19). After cisplatin treatment, the inhibition of cell proliferation and suppression of colony formation were reduced in osteosarcoma cells expressing CD74. Interestingly, the effect of inhibiting anchorage independent growth was activated after cisplatin treatment in the cells with very high expression CD74 mRNA.
REFERENCES
1. Kager L, Zoubek A, Pötschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003; 21:2011–8.

2. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003; 3:791–800.

3. Wilson KM, Labeta MO, Pawelec G, Fernandez N. Cell-surface expression of human histocompatibility leucocyte antigen (HLA) class II-associated invariant chain (CD74) does not always correlate with cell-surface expression of HLA class II molecules. Immunology. 1993; 79:331–5.
4. Imanishi T, Abe Y, Suto R. . Expression of the human multidrug resistance gene (MDR1) and prognostic correlation in human osteogenic sarcoma. Tokai J Exp Clin Med. 1994; 19:39–46.
5. Lazova R, Moynes R, May D, Scott G. LN-2 (CD74). A marker to distinguish atypical fibroxanthoma from malignant fibrous histiocytoma. Cancer. 1997; 79:2115–24.
6. Borghese F, Clanchy FI. CD74: an emerging opportunity as a therapeutic target in cancer and autoimmune disease. Expert Opin Ther Targets. 2011; 15:237–51.

7. Hira E, Ono T, Dhar DK. . Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer. 2005; 103:588–98.

8. Meyer-Siegler KL, Ordorica RC, Vera PL. Macrophage migration inhibitory factor is upregulated in an endotoxin-induced model of bladder inflammation in rats. J Interferon Cytokine Res. 2004; 24:55–63.

9. Grimer RJ, Bielack S, Flege S. . Periosteal osteosarcoma: a European review of outcome. Eur J Cancer. 2005; 41:2806–11.
10. Cesari M, Alberghini M, Vanel D. . Periosteal osteosarcoma: a single-institution experience. Cancer. 2011; 117:1731–5.
11. Han I, Lee MR, Nam KW, Oh JH, Moon KC, Kim HS. Expression of macrophage migration inhibitory factor relates to survival in high-grade osteosarcoma. Clin Orthop Relat Res. 2008; 466:2107–13.

12. Tarnowski M, Grymula K, Liu R. . Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res. 2010; 8:1328–43.

13. Seike T, Fujita K, Yamakawa Y. . Interaction between lung cancer cells and astrocytes via specific inflammatory cyto-kines in the microenvironment of brain metastasis. Clin Exp Metastasis. 2011; 28:13–25.

Figure 1
Representative microscopic images of CD74 and macrophage migration inhibitory factor (MIF) by immunohistochemistry (×200). (A) Normal bone tissue. (B) Low CD74 and low MIF expression. (C) High CD74 and high MIF expression.
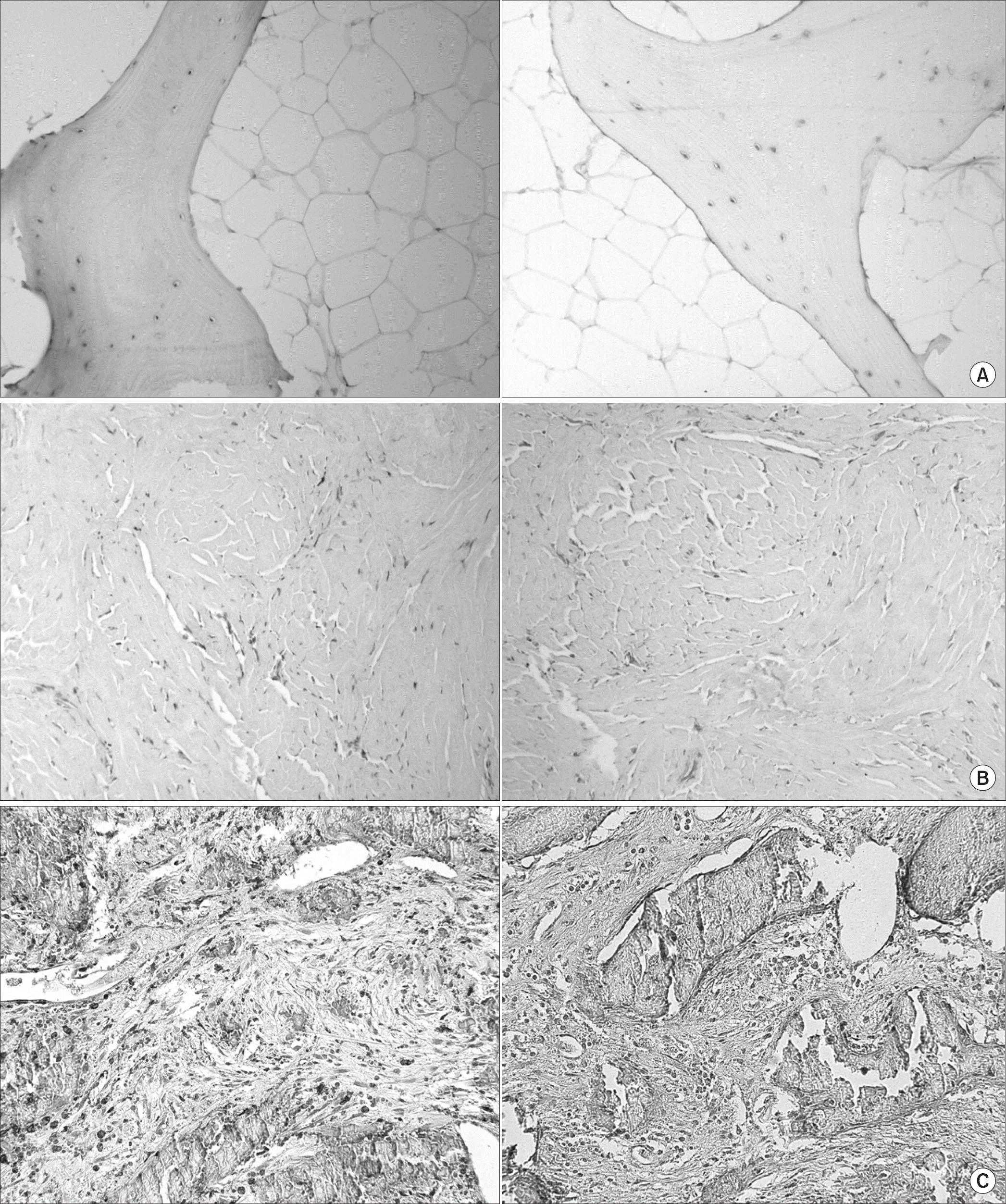
Figure 2
mRNA expression of CD74 in 22 patient-derived osteosarcoma primary cells. The CD74 mRNA level was significantly higher in all 22 cells than human osteoblast cell line (hFob 1.19; standard[s]) and osteosarcoma cell line (MG63) (p<0.05).
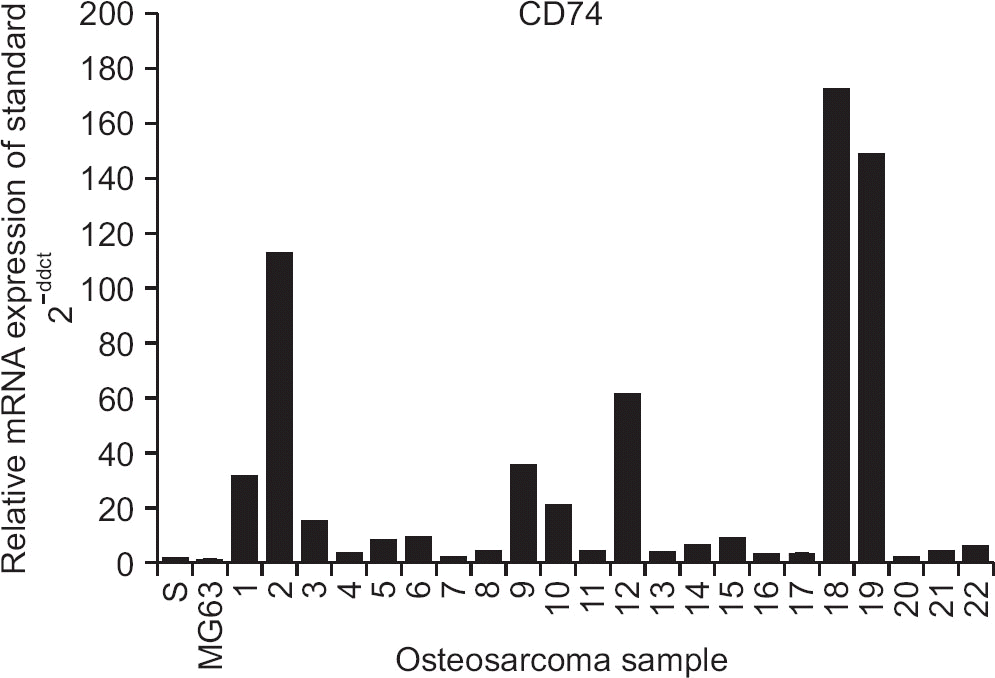
Figure 3
Relationship between CD74 expression and the effect of cisplatin on osteosarcoma proliferation. The effect of cisplatin is reduced in osteosarcoma cells with high CD74 expression (patient derived osteosarcoma primary cell) compared with osteosarcoma cells (MG63) with low CD74 expression. *p<0.05.
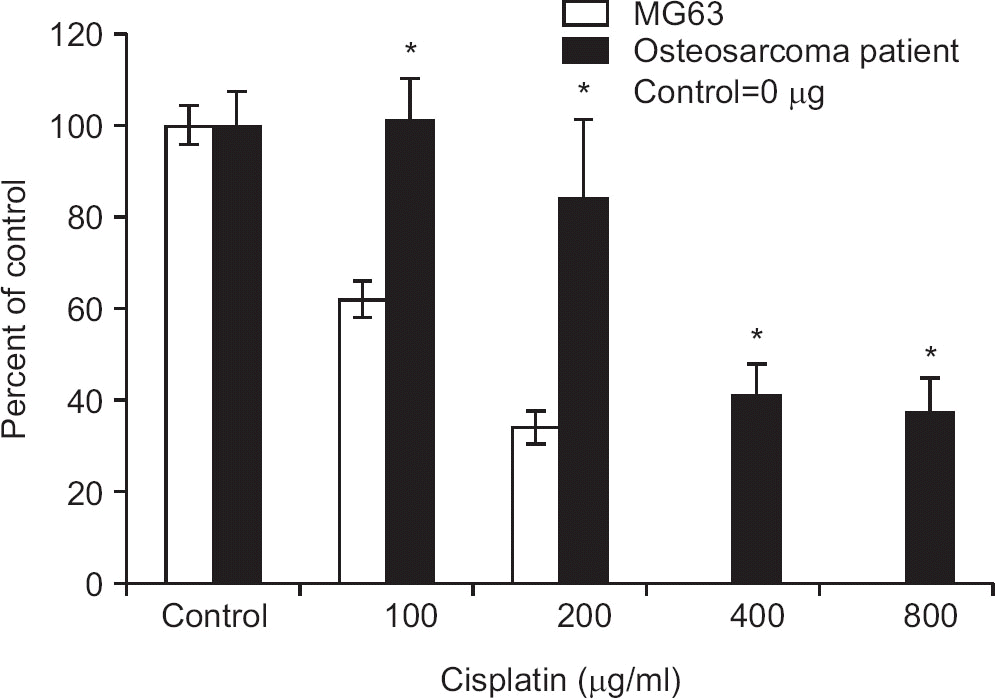
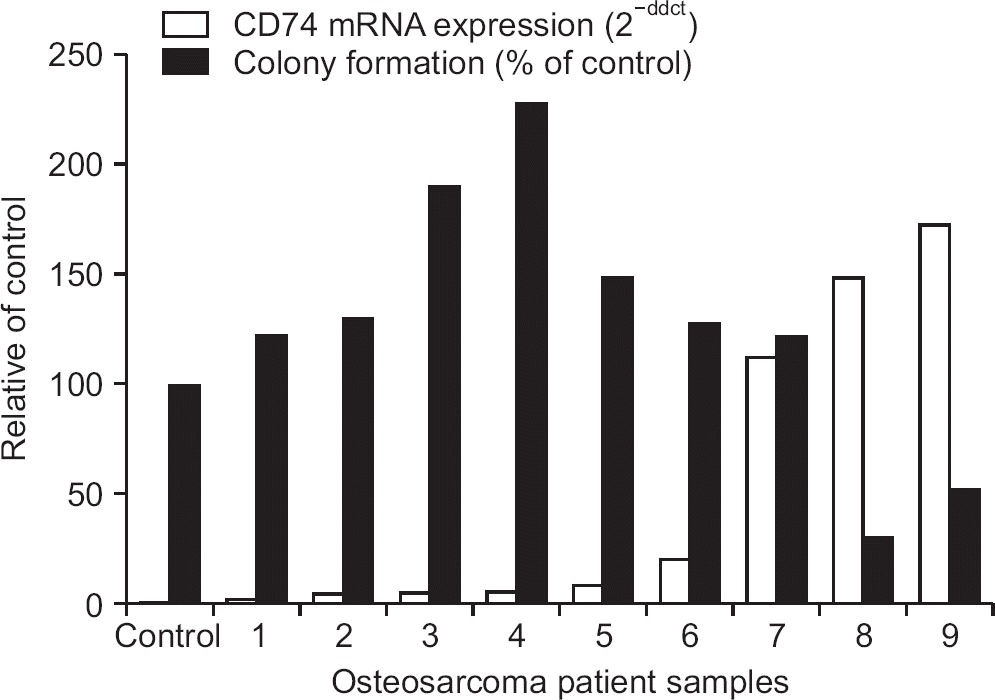
Figure 4
Relationship between colony counts by colony forming assay and CD74 mRNA expression by real-time polymerase chain reaction. Osteosarcoma cells of patients with high CD74 mRNA expression (□; 2-ddct) showed a marked decrease in colony formation (■; % of control) by cisplatin treatment (100 μg), indicating increased effect on anchorage independent growth.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download