Abstract
Septic arthritis of the hip is rarely caused by Mycoplasma hominis. It rarely develops in a patient during the postpartum period. However, delayed treatment of septic arthritis of the hip may lead to serious sequelae; therefore, it is important for clinicians not to overlook patients with the disease. This case illustrates the clinical steps in diagnosis and treatment of M. hominis septic arthritis of the hip.
Septic arthritis of the hip is an orthopaedic emergency because delayed treatment may lead to serious sequelae, including avascular necrosis of the femoral head, proximal femoral and/or pelvic osteomyelitis, and systemic sepsis.1) Mycoplasma hominis is common in the human genitourinary tract, and it is generally associated with localized urogenital tract infections, including pelvic inflammatory diseases, pyelonephritis, chorioamnionitis, and postpartum fever.2) M. hominis, however, is an extremely rare cause of septic arthritis, which is the reason many clinicians often miss the diagnosis and thus delay the timely treatment.3) We describe a case of septic arthritis caused by M. hominis occurring in the postpartum period in a relatively healthy woman.
A 29-year-old woman (gravida 1) came to the hospital with severe left hip pain and inability to bear weight 7 days after delivery. She had been admitted to another hospital at intrauterine pregnancy (IUP) 30 weeks and had been treated with uterine relaxant (eg. Ritodrine hydrochloride) to control prematurity. Experiencing prolonged rupture of the membranes at IUP 34 weeks, she received a caesarean section for a breech presentation. Although febrile on admission (a temperature of 39℃), she did not appear ill. On the physical examination, active and passive movements were limited because of the pain, particularly during internal rotation. The laboratory findings revealed an elevated white cell count of 13,700/µl with 78% neutrophils and 15% lymphocytes, C-reactive protein 8.75 mg/dl, erythrocyte sedimentation rate 83 mm/h. A magnetic resonance imaging of the hip joint (Fig. 1) confirmed a moderate joint effusion with surrounding soft tissue edematous change. Based on the above, septic arthritis was strongly suspected. Arthrocentesis of the joint yielded 5 ml of slightly turbid and non-thick fluid which revealed pus cells (white blood cell [WBC] 20,500/µl, segmented neutrophil 95%, glucose 2 mg/dl) but no micro-organisms on gram stain. Because her clinical presentation and laboratory findings were not correlated to the typical septic arthritis, we suspected other pathogens such as mycoplasma and virus which can lead to insidious onset and low-grade symptom presentation. A subsequent aerobic culture, an anaerobic culture, and Mycofast Evolution 2 test (International Microbio, Signes, France) were carried out. We irrigated the hip joint using two spinal needles (16 gauge) with 3,000 ml of sterile saline without antibiotics mixed. Postoperative drains were not used (Fig. 2). The patient received the empirical antibiotic treatment with IV 1st generation cephalosporin (1 g; TID) and aminoglycoside (240 mg; QD). Despite the hip joint irrigation and empirical antibiotics, the patient's clinical conditions did not improve; persistent fever (intermittent temperature 38℃), elevated inflammatory markers (C-reactive protein 12.56 mg/dl, erythrocyte sedimentation rate 105 mm/h) and hip pain still presented. Left hip joint irrigation was carried out again after 2 days, which showed aggravated joint fluid analysis results (WBC 35,000/µl, segmented neutrophil 86%, glucose 3 mg/dl, more turbidity). On this day, the Mycofast test of the joint fluid showed a positive result. Consequently antibiotic treatment was changed to IV lincomycin (500 mg; BID) and oral ciprofloxacin (500 mg; BID) for 2 weeks. The patient recovered an almost normal range of movement of the hip and was discharged with oral antibiotics (clindamycin 150 mg; TID and ciprofloxacin 500 mg; BID for 6 weeks). The relationship between the trend of the patient's inflammatory markers, fever pattern, and antibiotic prescription is shown in Fig. 3.
Septic arthritis caused by M. hominis infection has rarely been reported in the literature (about 27 cases). Interestingly, of the 27 cases, four have occurred in the postpartum period.3,4,5) According to Luttrell et al.,4) they found that the most commonly affected area was around the knee or the hip (50% and 33% of cases, respectively). Infection around the spine, shoulder, wrist and ankle was also reported. The precipitating causes were postpartum, recent genitourinary surgery, manipulation or immunocompromised conditions (e.g. hypogammaglobulinemia, solid organ transplant, collagen vascular disease or hematologic malignancy).
Mycoplasmas are the smallest free-living microorganisms. Being undetectable by Gram stain due to the lack of a cell wall, the organism is difficult to identify. In addition, their characteristic slow growth on routine blood culture media often leads to a considerable delay in diagnosis.2) Recently, helping to minimize this delay, commercial media have been developed, such as Mycofast Evolution 2 (International Microbio) and Mycoplasma IST 2 (BioMerieux, Marcy L'Etoile, France). They are designed for detection, enumeration and identification of Ureaplasma urealyticum and M. hominis in endocervical, urethral, urinary, gastric and sperm specimens. These media identify U. urealyticum and M. hominis growth after 24 hours of incubation in a liquid medium. Park et al.6) reported that the Mycofast test showed a sensitivity and specificity of 95%, 98%, respectively, and a positive and negative predictive value of 96%, 97%, respectively.
A synovial fluid examination is essential for the diagnosis of septic arthritis, gout, pseudogout, etc. Shmerling7) found that 89% of their septic arthritis patients with a total WBC count >50,000/µl had the septic joint. But other studies revealed that less than 50,000/µl of synovial WBC could not reliably exclude the possibility of a septic arthritis.7,8) Our case (WBC 20,500/µl, segmented neutrophil 95%, glucose 2 mg/d) was also line with those reports. Therefore, it is important not to overlook septic arthritis that does not have a typical finding of synovial joint fluid.
Recently, arthroscopic surgery of the hip for septic arthritis is being performed because it may have potential advantages over the traditional open arthrotomy. But Clarke et al.9) reported an overall complication rate of arthroscopic surgery as 1.4%; the complications involved neuropraxia of femoral and sciatic nerves, portal wound bleeding, portal hematoma, trochanteric bursitis and instrument breakage. In our case, to irrigate the hip joint, we used the two-needle irrigation technique under a local anesthesia instead of arthroscopic irrigation. This method could be a simpler and less invasive technique than arthroscopic surgery.
There is currently a lack of consensus for the optimal treatment for M. hominis septic arthritis. Mycoplasmas as a whole are innately resistant to certain antibiotics, such as penicillin, cephalosporin and rifamycin. Furthermore, M. hominis is innately resistant to erythromycin to which other species are sensitive. Historically, tetracyclines have been the first-line agents. However, since the proportion of M. hominis strains that are resistant to tetracyclines has been increasing (20%), other antibiotics such as lincomycin, clindamycin or fluoroquinolones (often ofloxacin) may sometimes need to be used.2)
The optimal duration of treatment for septic arthritis caused by M. hominis is also not well established due to the relative rarity of such infection. Clinical results were reviewed by Luttrell et al.,4) and they found treatments for septic arthritis by using joint drainage and antibiotics for ranging between 6 weeks and 4 months. In our case, since the patient did not feel pain around the hip joint during activities of daily living and showed the normal range of serologic markers for two consecutive times, we discontinued using the antibiotics 8 weeks after the first treatment. After discontinuing antibiotics, the patient was followed up for additional 6 months and the level of serologic markers was lower than the normal range.
Finally, we conjectured about why the patient was affected during the postpartum period. One possible cause is the alteration of immunity during the postpartum period. The immunologic recovery triggered by rapid resolution of pregnancy-associated immunosuppression may tip the balance in favor of a proinflammatory state and could potentially lead to deleterious consequences.10)
In summary, in the postpartum period if a patient's clinical symptoms and laboratory findings are assumed to be septic arthritis but are not typical characteristics of septic arthritis, we should suspect and perform specific tests for M. hominis infection. If M. hominis infection is diagnosed, then an early two-needle irrigation technique with proper antibiotics would be a simple and effective course of treatment, leading to positive results.
Figures and Tables
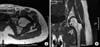 | Figure 1Magnetic resonance imaging (MRI) of the hip joint. Axial (A) and coronal (B) MRI images of the hip joint show a moderate joint effusion with edematous change on the surrounding soft tissue. |
References
1. Burton JH. Acute disorders of the joints and bursae. In : Tintinalli JE, Kelen GD, Stapezynski JS, editors. Emergency medicine: a comprehensive study guide. 6th ed. Tintinalli, New York: Mcgraw-Hill;2004. p. 1795–1801.
2. Taylor-Robinson D, Bébéar C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997; 40:622–630.

3. Phuah CL, Javid B, Aliyu SH, Lever AM. A case of Mycoplasma hominis septic arthritis postpartum. J Infect. 2007; 55:e135–e137.

4. Luttrell LM, Kanj SS, Corey GR, et al. Mycoplasma hominis septic arthritis: two case reports and review. Clin Infect Dis. 1994; 19:1067–1070.

5. Lee JH, Lee JH, Lee NY, Ha CW, Chung DR, Peck KR. Two cases of septic arthritis by Mycoplasma hominis after total knee replacement arthroplasty. Korean J Lab Med. 2009; 29:135–139.

6. Park HR, Kim YH, Lee HJ, Oh JS, Kim HJ. Usefulness of the Mycofast test (MYCOFAST(R) Evolution 2) for the diagnosis of nongonococcal genitourinary infections. Korean J Urol. 2006; 47:1117–1123.
7. Shmerling RH. Synovial fluid analysis. A critical reappraisal. Rheum Dis Clin North Am. 1994; 20:503–512.
8. McGillicuddy DC, Shah KH, Friedberg RP, Nathanson LA, Edlow JA. How sensitive is the synovial fluid white blood cell count in diagnosing septic arthritis? Am J Emerg Med. 2007; 25:749–752.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download