Abstract
Purpose
We analyzed the usefulness of vacuum-assisted closure (VAC) dressing to facilitate the healing of difficult wounds by comparing the results of conventional dressings.
Materials and Methods
We selected 20 cases for the experimental group (VAC group) and 20 cases for the control group (conventional dressing), and investigated the change in wound size, formation of granulation tissue, and duration of wound healing in the two groups.
Results
In the VAC group, the size of wound decreased from 60.2±59.1 cm2 to 29.7±18.8 cm2 (p=0.001). In the control group, it decreased from 60.3±83.3 cm2 to 34.4±47.6 cm2 (p=0.04). For formation of granulation, it increased from 1.2±0.4 to 2.7±0.6 (p=0.001) in the VAC group and from 1.2±0.4 to 2.4±0.5 in the control group. For the duration of healing, it took 17.5±8.3 days for the VAC group and 22.9±22.0 days in the control group (p=0.857). However there were no statistically significant differences in all the parameters between the 2 groups (p>0.05).
Conclusion
The clinical application of VAC to difficult wound yield comparable results in terms of a decrease in wound size, formation of granulation, and the duration of healing. VAC dressing could be an alternative treatment option for a difficult wound considering the advantage of saving medical human resources.
Figures and Tables
 | Figure 1Comparison of the wound size between the CD group and the VAC group. VAC, vacuum-assisted closure; CD, conventional dressing. |
 | Figure 2Changes in the grade of granulation between the CD group and the VAC group. VAC, vacuum-assisted closure; CD, conventional dressing. |
 | Figure 3The difference in the period of dressing between the CD group and the VAC group. VAC, vacuum-assisted closure; CD, conventional dressing. |
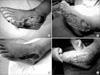 | Figure 4Clinical application of VAC for a 70-year-old female with diabetes mellitus foot (A) about 15×10 cm sized necrotic soft tissue infection developed on the left foot due to burn. (B) Debridement was performed. (C) After application of VAC for 2 weeks, the size of defect decreased to 10×3 cm. (D) Then, a full thickness skin graft was performed. VAC, vacuum-assisted closure. |
References
1. Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997. 38:563–576.
2. Harrah J, Gates R, Carl J, Harrah JD. A simpler, less expensive technique for delayed primary closure of fasciotomies. Am J Surg. 2000. 180:55–57.

3. Hussmann J, Kucan JO, Zamboni WA. Elevated compartmental pressures after closure of a forearm burn wound with a skin-stretching device. Burns. 1997. 23:154–156.

4. Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989. 109:317–330.

5. Pietramaggiori G, Liu P, Scherer SS, et al. Tensile forces stimulate vascular remodeling and epidermal cell proliferation in living skin. Ann Surg. 2007. 246:896–902.

6. Van der Velde M, Hudson DA. VADER (vacuum-assisted dermal recruitment): a new method of wound closure. Ann Plast Surg. 2005. 55:660–664.
7. Frame JD, Still J, Lakhel-LeCoadou A, et al. Use of dermal regeneration template in contracture release procedures: a multicenter evaluation. Plast Reconstr Surg. 2004. 113:1330–1338.

9. Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999. 7:442–452.

10. Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen. 2000. 8:347–352.
11. Song DH, Wu LC, Lohman RF, Gottlieb LJ, Franczyk M. Vacuum assisted closure for the treatment of sternal wounds: the bridge between débridement and definitive closure. Plast Reconstr Surg. 2003. 111:92–97.

12. Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997. 38:553–562.
13. Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijil JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006. 118:390–397.

14. Fitzgerald AM, Gaston P, Wilson Y, Quaba A, McQueen MM. Long-term sequelae of fasciotomy wounds. Br J Plast Surg. 2000. 53:690–693.

15. Zannis J, Angobaldo J, Marks M, et al. Comparison of fasciotomy wound closures using traditional dressing changes and the vacuum-assisted closure device. Ann Plast Surg. 2009. 62:407–409.

16. Taylor RC, Reitsma BJ, Sarazin S, Bell MG. Early results using a dynamic method for delayed primary closure of fasciotomy wounds. J Am Coll Surg. 2003. 197:872–878.

17. Hildebrand F, Giannoudis P, Kretteck C, Pape HC. Damage control: extremities. Injury. 2004. 35:678–689.

18. Roberts CS, Pape HC, Jones AL, Malkani AL, Rodriguez JL, Giannoudis PV. Damage control orthopaedics: evolving concepts in the treatment of patients who have sustained orthopaedic trauma. Instr Course Lect. 2005. 54:447–462.
19. Brandi C, Grimaldi L, Nisi G, et al. Treatment with vacuum-assisted closure and cryo-preserved homologous de-epidermalised dermis of complex traumas to the lower limbs with loss of substance, and bones and tendons exposure. J Plast Reconstr Aesthet Surg. 2008. 61:1507–1511.

20. Kang GC, Por YC, Tan BK. In vivo tissue engineering over wounds with exposed bone and tendon: autologous dermal grafting and vacuum-assisted closure. Ann Plast Surg. 2010. 65:70–73.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download