Abstract
Purpose
We observed new bone formation following the transplantation of allogenic periosteum-derived stem cells and different sizes of hydroxyapatite (HA) scaffold materials into rabbit long-bone defects.
Materials and Methods
Thirty-two white rabbits were grouped according to the material transplanted into their tibial bone defects: group 1 (microscale HA only); group 2 (nanoscale HA only); group 3 (microscale HA plus stem cells); and group 4 (nanoscale HA plus stem cells). Viscosity was controlled by the relative amounts of HA and agar. After surgery, radiologic, microscopic, and biochemical observations were performed weekly for 8 weeks.
Figures and Tables
 | Figure 1An external fixation device was applied to the rabbit tibia (A). After exposure of the tibia (B), an 1.5 cm section of the tibia was excised (C). A mixture of agar, hydroxyapatite powder, and stem cells was inserted into the bone defect (D). |
 | Figure 4(A) HA nanoparticles attached on the surface of periosteum-derived stem cell. (B) The effect of particle volume fraction on the ratio of the viscosity of agar based nanofluid to that of base fluid. |
 | Figure 5Serial radiographs of groups 1 through 4. In group 1 (A), diffuse bone formation was seen at 3 weeks and consolidation was complete after 8 weeks, but only in the lateral portion of the defect. In groups 2 (B), 3 (C), and 4 (D), bone formation and consolidation were seen as time passed. More abundant bone formation was seen in the groups transplanted with stem cells (C, D). |
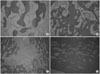 | Figure 6Microscopic findings of new bone at 8 weeks after transplantation (H-E, ×100). (A) In group 1, cartilaginous components were predominant. (B) In group 2, well-formed lamellar bone was observed. (C) In group 3, defects showed mostly loose connective tissue, with scant amounts of woven and lamellar bones. (D) In group 4, the tibial defects were almost filled with inflammatory cells, with conspicuous bone formation. |
 | Figure 7Biweekly changes in optical density of alkaline phosphatase (ALP) for groups 1 through 4. Optical density of ALP was measured at 405nm. Control values were measured 1 day before surgery; values for control through 6 weeks are the means obtained from 5 rabbits. |
 | Figure 8Biweekly changes in optical density of osteocalcin (OC) for groups 1 through 4. Optical density of OC was measured at 450nm. Control values were measured 1 day before surgery; values for control through 6 weeks are the means obtained from 5 rabbits. |
References
2. Nkenke E, Schultze-Mosgau S, Radespiel-Tröger M, Kloss F, Neukam FW. Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res. 2001. 12:495–502.

3. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004. 25:4731–4739.

4. Du C, Cui FZ, Zhu XD, de Groot K. Three-dimensional nano-HAp/collagen matrix loading with osteogenic cells in organ culture. J Biomed Mater Res. 1999. 44:407–415.

5. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000. 21:1803–1810.

6. Hamanishi C, Yoshii T, Totani Y, Tanaka S. Bone mineral density of lengthened rabbit tibia is enhanced by transplantation of fresh autologous bone marrow cells. An experimental study using dual X-ray absorptiometry. Clin Orthop Relat Res. 1994. (303):250–255.
7. Heraeus Kulzer. Ostim-Vollsynthetischer Knochenersatz "ready to use". Das Deutsche Zahnarzteblatt. 2003. 5:67.
8. Jang SP, Lee JH, Hwang KS, Choi SUS. Particle concentration and tube size dependence of viscosities of Al2O3-water nanofluids flowing through micro- and minitubes. Appl Phys Lett. 2007. 91:243112.
9. LeGeros RZ. Apatites in biological systems. Prog Cryst Growth Charact. 1981. 4:1–45.
10. LeGeros RZ. Calcium phosphate materials in restorative dentistry: a review. Adv Dent Res. 1988. 2:164–180.

11. Kim HW, Knowles JC, Kim HE. Hydroxyapatite and gelatin composite foams processed via novel freeze-drying and crosslinking for use as temporary hard tissue scaffolds. J Biomed Mater Res A. 2005. 72:136–145.

12. Yaylaoğlu MB, Korkusuz P, Ors U, Korkusuz F, Hasirci V. Development of a calcium phosphate-gelatin composite as a bone substitute and its use in drug release. Biomaterials. 1999. 20:711–719.
13. Schwartz Fo HO, Novaes AB Jr, de Castro LM, Rosa AL, de Oliveira PT. In vitro osteogenesis on a microstructured titanium surface with additional submicron-scale topography. Clin Oral Implants Res. 2007. 18:333–344.

15. Colon G, Ward BC, Webster TJ. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res A. 2006. 78:595–604.
16. Thorwarth M, Schultze-Mosgau S, Kessler P, Wiltfang J, Schlegel KA. Bone regeneration in osseous defects using a resorbable nanoparticular hydroxyapatite. J Oral Maxillofac Surg. 2005. 63:1626–1633.

17. Yaakobi T, Maltz L, Oron U. Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He-Ne) irradiation. Calcif Tissue Int. 1996. 59:297–300.

18. Yoo JU, Johnstone B. The role of osteochondral progenitor cells in fracture repair. Clin Orthop Relat Res. 1998. 355 Suppl. S73–S81.

19. Obrant KJ, Merle B, Bejui J, Delmas PD. Serum bone-gla protein after fracture. Clin Orthop Relat Res. 1990. (258):300–303.

20. Ohishi T, Takahashi M, Kushida K, et al. Changes of biochemical markers during fracture healing. Arch Orthop Trauma Surg. 1998. 118:126–130.

21. Kim HW, Kim HE, Salih V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials. 2005. 26:5221–5230.

22. Elias KL, Price RL, Webster TJ. Enhanced functions of osteoblasts on nanometer diameter carbon fibers. Biomaterials. 2002. 23:3279–3287.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download